1. Ayushman Bharat: Ayushman Bharat comprises of two components:
a. Ayushman Arogya Mandir
The first component pertains to creation of 1,50,000 Health and Wellness Centres (AB-HWCs), now renamed as Ayushman Arogya Mandir, by upgrading the Sub Health Centres (SHCs) and rural and urban Primary Health Centres (PHCs), in both urban and rural areas, to bring health care closer to the community. These centres aim to provide Comprehensive Primary Health Care (CPHC), by expanding and strengthening the existing Reproductive & Child Health (RCH) and Communicable Diseases services and by including services related to Non-Communicable Diseases (common NCDs such as, Hypertension, Diabetes and three common cancers of Oral, Breast and Cervix) and incrementally adding primary healthcare services for mental health, ENT, Ophthalmology, Oral health, Geriatric and Palliative care and Trauma care as well as health promotion and wellness activities like yoga. A few States/UTs have already started rolling out these additional packages in a phased manner.
Comprehensive Primary Health Care (CPHC) through Ayushman Arogya Mandir – Ayushman Bharat aims to holistically address health (covering preventive, promotive, curative, rehabilitative and palliative care), at primary, secondary and tertiary level by adopting a continuum of care approach. In the lifetime of an individual, the primary healthcare services cater to 80- 90% of the healthcare needs for improved healthcare outcomes and quality of life of the population.
The Primary Health Care team ensures that community outreach and population enumeration are done for individuals in their catchment area and screened for communicable diseases and non-communicable diseases for early detection and timely referral for accurate diagnosis. The team further ensures that treatment adherence and follow-up care are provided to the patients in the community. The essential health services along with the provisioning of essential medicines and diagnostics are provided closer to the community through these centres, as a step towards building stronger and resilient primary healthcare systems which cater to the healthcare needs of the population.
b)Ayushman Bharat PM-JAY:
- Ayushman Bharat Pradhan Mantri – Jan Arogya Yojana (AB PM-JAY) is the largest publicly funded health assurance scheme in the world which provides health cover of Rs. 5 lakhs per family per year for secondary and tertiary care hospitalization.
- Currently, 55 Crore individuals corresponding to 12 Crore families are covered under the scheme. Many States/UTs implementing AB PM-JAY have further expanded the beneficiary base, at their own cost.
- As of 20th December 2023, approximately 28.45 Crore Ayushman Cards have been created since the inception of the scheme, out of which, approximately 9.38 crore Ayushman Cards have been created during the current year 2023 (Jan -Dec 2023).
- A total of 6.11 crore hospital admissions amounting to Rs. 78,188 crores have been authorized under the scheme, of which 1.7 crore hospitals admissions worth over Rs. 25,000 crores have been authorized during the year 2023 (Jan-Dec 2023).
- A total of 26,901 hospitals including 11,813 private hospitals have been empanelled under AB PM-JAY to provide healthcare services to scheme beneficiaries.
- AB PM-JAY has ensured gender equity in access to healthcare services.
- Women account for approximately 49% of the total Ayushman cards created and approximately 48% of total authorized hospital admissions.
- Ayushman Bhava initiative was launched by MoHFW for ensuring the saturation of various healthcare schemes at the village level, ultimately reaching the last mile beneficiaries. It was launched by Hon’ble President Smt. Droupadi Murmu on 13th September 2023.
- This initiative encompasses a series of interventions, including ‘Aapke Dwar Ayushman 3.0’, ‘Ayushman Sabhas’, ‘Ayushman Melas’, and the ultimate goal of elevating villages to the status of ‘Ayushman Gram’.
- NHA launched ‘Aapke Dwar Ayushman’ (ADA 3.0) campaign on 17th September 2023 as part of Ayushman Bhava campaign.
- An Android based ‘Ayushman App’ has been launched by National Health Authority wherein self-verification feature for beneficiaries has been enabled. The app has been developed using latest technology and provided different modes of authentication i.e. face-auth, OTP, IRIS, and fingerprint for Ayushman Card creation.
- This ensures that any mobile device can be used for Ayushman card creation. As a result, as of 20th December 2023, approximately 3.85 crore verification for Ayushman Card creation have been done during Ayushman Bhava campaign.
- Viksit Bharat Sankalp Yatra (VBSY) has been launched by Hon’ble Prime Minister on 15th November 2023.
- The purpose of the Yatra is to raise awareness about the government’s development policies and schemes among the people, provide benefits of the scheme to eligible population and foster an atmosphere of trust and collaboration.
- Ayushman Card creation and delivery have been selected for the on-spot services to be offered during the Yatra. As of 20th December 2023, a total of 96.03 lakh Ayushman Cards have been created during the campaign.
1.1 Achievement and Service Delivery at Ayushman Arogya Mandir:
- As reported by the States/UTs on the Ayushman Arogya Mandir Portal, 1,63,402 Ayushman Arogya Mandir have been operationalized till 15th December, 2023.
- As per the data update done by the States/UTs in Ayushman Arogya Mandir Portal, till date, 55.66 crore screenings have been done for hypertension and 48.44 crore screenings done for diabetes. Similarly, these functional Ayushman Arogya Mandir have done 32.80 crore screenings for oral cancer, 14.90 crore screenings for cervical cancer in women and more than 10.04 screenings for breast cancer in women.
- Further, as on 15th December, 2023, a total of 2.80 crore Yoga/wellness Sessions have been conducted in operational Ayushman Arogya Mandir.
- With the objective to provide quality health services to a patient residing in rural areas, Ayushman Arogya Mandir have facility of tele- consultation, achieving a total of more than 17 crore teleconsultations so far.
- Viksit Bharat Sankalp Yatra
lndia is a country that is constantly progressing and growing with 1.4 billion population both in rural and urban areas. Since 2014, the Government of lndia has been committed to a model of development that ensures no one is left behind, “Sabka Sath Sabka Vikas, Sabka Vishwas, Sabka Prayaas”. To celebrate last 9 years’ achievements, in various fields across various schemes Government of lndia is conducting a nationwide awareness campaign named as “Viksit Bharat Sankalp Yatra”. The campaign is designed to build on accomplishments of Government of lndia and usher in an era collective participation where rural and urban communities can actively engage in the nation’s development. A 60-days long Yatra is being organised covering all districts, Gram Panchayat and urban locations across the country from 3rd week of November 2023 to January 2024.
The major objectives of Yatra include reaching the unreached especially the vulnerable population, dissemination of information about the schemes through Mobile vans travelling across the country, Enrolment of potential beneficiaries under various schemes and learning from citizens through interaction with beneficiaries of government schemes by personal stories/experience sharing.
Ministry of Health and Family Welfare (MoHFW), has identified Schemes/programs to be showcased during the course of the Yatra such as Ayushman Bharat- Pradhan Mantri Jan Arogya Yojana as the Flagship Scheme of MoHFW and national TB Elimination Programme for the campaign with additional focus on Sickle Cell Anaemia Elimination Mission in Tribal Area. Further, Health Camps are also being organised at the places of halt of the mobile van where in Screening and Referral for Tuberculosis, Non-Communicable Diseases and Sickle Cell Disease, Nikshay Mitra Registration and Consent under Nikshay Mitra, Seeding of Bank Accounts for Nikshay Poshan Yojna, Ayushman Card creation and Physical Card distribution are conducted.
MoHFW has constituted a Central Co-ordination Committee along with senior officials being nominated as State Nodal Officers to oversee the activities of Viksit Bharat Sankalp Yatra.Also, 169 District Health Nodal Officers from DGHS, Regional Offices of Health and Family Welfare (RoHFW), National Centre for Disease Control (NCDC) have been given a checklist to ensure that requisite preparations have taken place and have also been requested to visit the district at least one day prior to the start of Yatra.Control Room has also been established to support States. Sequence of trainings and meetings have been conducted with the States to orient and monitor the progress on regular basis. Data Entry Microsite has been developed by MoHFW to capture the data real time.
Till 30th November,2023 information pertaining to 12,774 Gram Panchayats have been received with the total footfall of more than 18,24,582 people. 18,05,069 Ayushman Cards have been created and 3,20,872 Ayushman cards have been physically distributed. 5,91,491 people have been screened for Tuberculosis, out of which more than 47,189 were referred to higher Public Health Facilities. 88,041 people have been screened for Sickle Cell Disease, out of which 3,995 were found to be positive and referred to higher Public Health Facilities. Around 6,99,308 people were screened for Hypertension and 6,52,101 people screened for Diabetes. More than 70,281 people were suspected to be positive for Hypertension and more than 52,149 were suspected to be positive for Diabetes and more than 1,13,706 people were referred to higher Public Health Facilities.
- National COVID-19 Vaccination Programme
On 16th January 2021, India launched the National COVID-19 Vaccination Programme. COVID vaccination in the country commenced with vaccination to all Health Care Workers followed by Front line Workers, population aged ≥60 years and has subsequently expanded to cover the population aged 12 years and above. The Vaccination Programme is being guided by immaculate planning based on a regular review of scientific and global test practices by National Expert Group on Vaccine Administration for COVID-19 (NEGVAC). Since the start of the COVID Vaccination drive, it has focused on taking decisions guided by science. Prioritizing our health workers, frontline workers and other vulnerable populations in a phased manner has been an excellent way to scale up the vaccination program. Vaccines are provided free of cost at all Government vaccination centres.
Under the National COVID-19 Vaccination Programme, three vaccines namely Covaxin manufactured by M/s Bharat Biotech International Limited, Covishield manufactured by M/s Serum Institute of India and CorBEvax manufactured by M/s Biological E. have been made available at the Government COVID-19 Vaccination Centres (CVCs). Additionally, Sputnik V vaccine developed by Gamaleya Research Institute, Russia (imported by Dr Reddy’s Lab), DNA–based ZyCov-D by M/s Cadila Healthcare Ltd., Covovax made by M/s Serum Institute of India, Gemcovac-19 and Gemcovac-OM by M/s Gennova Biopharmaceutical Limited and India’s first intranasal vaccine i.e iNCOVACC produced by M/s Bharat Biotech International Limited are available at private CVCs.
India was pioneer in developing and utilizing a digital platform – CoWIN (Winning over Covid) which acted as a backbone for implementation of the entire vaccination programme. The CoWIN not only proved to be a seamless digital delivery mechanism supporting the programme managers in vaccination session planning at vaccination centre level, inventory management, tracking of every unique vaccination event, line listing of due beneficiaries and coverage analysis etc., but also provided the beneficiaries with easy prior registration, advance booking of vaccination appointments as per each person’s preferences for the choice of vaccination centre, vaccination time slots and vaccine type, grievance redressal mechanism, availability of QR based individual digital certificates. With the administration of 200 crore Covid vaccine doses across the country on 17th July 2022, India achieved a significant milestone of administering over 100 crore doses of COVID vaccines to its eligible adult population in just 9 months and another set of 100 crores vaccine doses have been administered in the next 9 months, depicting sustainability. As on 21st December 2023, with 220.67 crore vaccine doses administered across the country, over 97% citizens have received the 1st dose of COVID-19 vaccine while over 90% eligible citizens have received 2nd dose of the vaccine. Additionally, 22.88 crore precaution doses have also been administered across the country among the eligible adult population.
- Immunization
• Introduction of third dose of fractional Inactivated Polio Virus (fIPV3): As recommended by the National Technical Advisory Group on Immunization (NTAGI), the third dose of fIPV has been included in the National Immunization schedule, effective from 1st January 2023. fIPV3 has to be administered intradermally, along with the MR containing vaccine during 9-12 months of age. It enhances the protective effects of the Polio vaccine.
• Polio Sub-National Immunization Day (SNID): India was declared Polio free Country in 2014 and to mitigate the risk of Polio myelitis from being transmitted from neighboring countries, regular Polio SNIDs are held in India. In the year 2023, 2 SNIDs have been conducted on 28th May 2023 and 10th Dec 2023 in high-risk areas in 13 States/UTs and over 200 identified districts throughout the country.
• Intensified Mission Indradhanush 5.0: The Intensified Mission Indradhanush (IMI) 5.0 is a catch-up vaccination campaign rolled out for children upto 5 years of age and pregnant women, who were left out or dropped out of routine immunization. Three rounds of IMI 5.0 were held in the month of August, September and October 2023, across all districts of the country with a special focus on Measles Rubella Elimination goal.
- Since 2014, 12 phases of Mission Indradhanush/IMI have been held across the country and so far 5.46 crore children and 1.32 crore pregnant women have been vaccinated.
• U-Win digital platform pilot
- Following the success of Co-WIN digital platform, an online case-based tracking and reporting system for the Universal Immunization Program, for both children and pregnant women was introduced in the month of January 2023, in a pilot mode across 65 districts nationwide, for registration of every vaccination event.
• Measles Rubella Elimination
- India is committed to Measles Rubella Elimination by December 2023. Robust MR surveillance across the country is being carried out with an all-time high NMNR (Non-Measles Non-Rubella discard rate) of 5.5/1 lakh population. Outbreak Investigations and MR supplementary vaccination campaigns is being carried out in various States/Districts where outbreaks have been reported to close the immunity gaps.
- National TB Elimination Programme (NTEP) Performance
With the goal of achieving Sustainable Development Goals related to TB by 2025, five years ahead of the global targets of 2030, the Ministry implements the National TB Elimination Programme with the following objectives:
- Early diagnosis of TB patients, prompt treatment with quality-assured drugs and treatment regimens.
- Engaging with the patients seeking care in the private sector.
- Prevention strategies include contact tracing in high-risk/vulnerable populations.
- Airborne infection control.
- Multi-sectoral response for addressing social determinants.
Achievements in Key Programme Indicators over the last 9 years are as under:
India pioneered a mathematical model for TB Burden Estimation, a first among nations, which shows that India is progressing against the SDG goals at a far greater pace than the global average, with a decline in TB incidence by 16% (as compared to the global decline of 10%) and a decline of 18% in TB deaths (as compared to the global decline of 10%).
- Pradhan Mantri TB Mukt Bharat Abhiyaan: Pradhan Mantri TB Mukt Bharat Abhiyaan was launched by the Honorable President of India on September 9, 2022, with the objectives to provide additional support to TB patients in order to improve treatment outcomes, augment community involvement and leverage Corporate Social Responsibility (CSR) activities. As per the clarion call of the Hon’ble Prime Minister of India, Shri Narendra Modi at Delhi End TB Summit in March 2018 to eliminate TB by 2025, five years ahead of Sustainable Development Goal, PMTBMBA initiative was launched to bring together people from all backgrounds into a ‘Jan Andolan’ and escalate the progress toward TB elimination.
Achievements under Pradhan Mantri TB Mukt Bharat Abhiyaan (PMTBMBA) (13.12.2023):
- Ni-kshay Mitra registered: 111,749.
- TB patients on treatment: 14.41 lakhs.
- TB patients consented to receive community support: 10.15 lakhs.
- Commitment by Ni-kshay Mitra for TB patients: 10.14 lakhs.
- Among the renowned Ni-kshay Mitras, Hon’ble Governors/Lt. Governors of 26 State/UTs, Hon’ble Union Ministers, Ministers of State, Chief Ministers, State Health Ministers of many State/UTs have come forward to adopt TB patients. Many MLAs & local parishads have also become Ni-kshay Mitras. Around 17 Cabinet Secretariat officials and 23 MoHFW/CTD officials have also adopted TB patients.
- TB Notifications: The overall notification of TB cases has improved by 64% over the last 9 years, from 2014 to 2023. India notified 24.2 lakh TB cases in 2022 which was higher than the pre-COVID level of 2019. In 2023, a total of 22.31 lakh TB patients have been notified till Nov 2023 (as on 01-12-2023). From 2015 onwards, the efforts to find all cases have resulted in over ~58% increase in TB case notifications.
- Private Sector Notification: With a focused and targeted engagement with the private sector through interventions like Patient Provider Support Agency (PPSA), gazette notification for mandatory notification of TB cases, incentives for notification of cases and collaborations with professional bodies like IMA, IAP, FOGSI, etc., there has been an increase in private sector notification by more than 7 times over the past 8 years. In 2022, the country was able to notify 7.33 lakh TB cases (highest ever) respectively accounting for 30% of total notifications. In 2023, till Nov, 7.3 lakh patients were notified from the private sector (as on 01-12-2023) which contributed to 32% of total notifications. The programmatic collaborative efforts resulted in a 7 times increase in cases reported from the private sector. The innovative private sector models have been global best practices.
- Introduction of newer anti-TB drugs – Bedaquiline, Delamanid: Shorter, safer oral Bedaquiline-containing DR-TB regimens have been rolled out pan-India across all states and UTs. These drugs are given to multi-drug-resistant TB patients with or without resistance to fluoroquinolones. In 2022, a total of 30,864 patients were initiated on the longer all-oral M/XDR-TB regimen and 27,431 patients were initiated on the shorter MDR/RR-TB regimen (Oral/Injection based). In 2023 till July, 39,186 patients have been diagnosed with MDR/RR and out of them 35,302 have been initiated on treatment (as on 17-08-2023). Amongst these, 17,836 patients were initiated on shorter oral MDR/RR-TB regimen (9-11 months) and 17,466 patients were initiated on longer M/XDR-TB regimen (18-20 months).
- TB Treatment Success Rate: Over the last 9 years, despite one-third of notifications coming from the private sector, the programme was able to sustain a treatment success rate of above 80%. In 2021, the success rate had reached 84% and in 2022, it marginally increased to 85.5%. In 2023 (till Nov), the success rate increased to 86.3%.
- Nikshay Poshan Yojana: Undernutrition is an important risk factor for TB, the Government introduced a scheme of Nikshay Poshan Yojana (NPY) in April 2018 for providing Rs 500/month as DBT to support the nutrition of TB patients for the entire duration of treatment. Cumulatively, till November-2023, NTEP has disbursed Rs 2617.44 Cr to 993 lakh TB patients.
- Active Case Finding: For reaching out to missing TB patients, the Government has begun systematic active TB case finding in high-risk groups. The programme has proactively conducted house-to-house searches of TB cases among these vulnerable populations. This includes people living with HIV, diabetics, undernourished, residential institutes like prisons, asylums, old age homes, orphanages, tribal areas, and marginalized populations. This activity has resulted in the diagnosis of an additional ~3 lakh TB cases since its inception.
- Infrastructure Scale-Up: There has been a huge infrastructure scale-up of TB laboratory services. Designated Microscopy Centers (DMCs) have increased by 80% (13583 in 2014 to 24449 in Nov 2023) over the past 9 years and 6196 new molecular diagnostic laboratories have been established till now. The number of drug-resistant TB treatment centers has increased from 127 in 2014 to 792 in 2022.
- Sub National disease-free certification: To monitor the trends of the TB Epidemic at the State/UTs/District level, the ministry has introduced a novel initiative of estimating disease burden through a methodology of community-level survey (Inverse sampling methodology) and tracking drug sales data in the private sector and measuring the level of under-reporting to the programme. Through this methodology, State/UTs/District level estimates of TB disease are derived and measured against the baseline of 2015.
In the year 2020, the State of Kerala, UTs of Lakshadweep, Puducherry and 35 districts have successfully achieved various levels of reduction in TB incidence. The UT of Lakshadweep and the district of Budgam in J&K were declared as the first UT & the first district in the country to achieve more than an 80% reduction in TB incidence. (SDG Targets).
In 2021, 3 States (Kerala, DNHDD & Puducherry) received Silver (>40% reduction) & 5 States (Gujarat, Himachal Pradesh, Sikkim, Tripura, Ladakh) received Bronze (>20% reduction). Whereas 8 districts receive Gold (>60% reduction), 27 districts received Silver & 56 districts received bronze.
In 2022, Karnataka received Silver (>40% reduction) and Jammu & Kashmir received Bronze (>20% reduction). Three districts were declared TB-free (>80% reduction), 17 districts received Gold (>60% reduction), 35 districts received Silver and 48 districts received Bronze.
Summary of achievements:
|
Indicators |
2014 |
2015 |
2016 |
2017 |
2018 |
2019 |
2020 |
2021 |
2022 |
Jan-Nov 2023 |
|
TB Notification (Lakhs) |
15.5 |
16.08 |
17.55 |
18.28 |
21.56 |
24.04 |
18.05 |
21.35 |
24.22 |
22.31 |
|
TB Notification-Private Sector (Lakhs) |
1.06 |
1.84 |
3.3 |
3.83 |
5.42 |
6.78 |
5.59 |
6.89 |
7.33 |
7.3 |
|
TB Treatment Success Rate |
81% |
87% |
78% |
79% |
81% |
81% |
82% |
84% |
85.5% |
86.3% |
|
Nikshay Poshan Yojana – DBT (Lakhs) (Beneficiaries paid at least one benefit) |
– |
– |
– |
– |
13.4 |
16.7 |
14.23 |
17.5 |
19.47 |
14.26* |
|
Active Case Finding (Additional Cases diagnosed) |
– |
– |
– |
– |
47307 |
62958 |
52273 |
73772 |
48953 |
17487 |
*-Till Nov 2023
|
Infrastructure |
2014 |
2015 |
2016 |
2017 |
2018 |
2019 |
2020 |
2021 |
2022 |
2023 (as on date) |
|
|
Designated Microscopy Centers |
13583 |
13886 |
13888 |
15307 |
16212 |
20356 |
21717 |
21820 |
23038 |
24449 |
|
|
Cartridge based Nucleic Acid Amplification Test (CBNAAT)/Truenat |
40 |
80 |
121 |
628 |
651 |
1135 |
3147 |
3760 |
5090 |
6196 |
|
- National AIDS and STD Control Programme (Phase-V) :2023
Introduction
- The Government of India is currently implementing the phase-V of the National AIDS and STD Control Programme (NACP) as a Central Sector Scheme fully funded by the Government of India from 1st April 2021 to 31st March 2026 with an outlay of Rs 15,471.94 Crore. The NACP phase-V anchors the national AIDS and STD response in the country till 2025-26 towards the attainment of the United Nations’ Sustainable Development Goal 3.3 of ending the HIV/AIDS epidemic as a public health threat through a comprehensive package of prevention, detection, and treatment services.
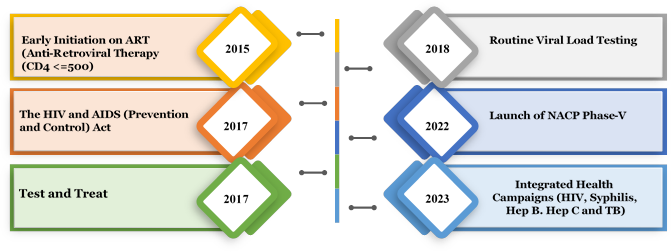
- NACP phase-V builds upon the game-changer initiatives undertaken during phase-IV including HIV/AIDS Prevention and Control Act (2017), test and treat Policy, universal viral load testing, mission Sampark, community-based screening and transition to Dolutegravir-based treatment regimen (Figure 1). NACP phase-V introduces newer strategies consolidating and augmenting the gains to attain the stated goal by 2025-26.
Figure 1. Ten years of game changers (2014-2023)
|
Early Initiation on ART (Anti-Retroviral Therapy (CD4 <=500) |
|
The HIV and AIDS (Prevention and Control) Act |
|
Test and Treat |
|
Routine Viral Load Testing |
|
Launch of NACP Phase-V |
|
Integrated Health Campaigns (HIV, Syphilis, Hep B. Hep C and TB) |
|
2015 |
|
2018 |
|
2017 |
|
2022 |
|
2017 |
|
2023 |
B. Prevention of new HIV infections
- The reduction of new HIV infections by 80% is the first goal of NACP phase-V. Continued and augmented focus on high-risk groups, bridge population and ‘at-risk’ populations is the mainstay of the NACP approach for the reduction of new HIV infections. Under the programme, approximately 14 to 15 lakh high-risk groups were covered in each of the first two years of NACP Phase-V. In 2023 till September, approximately 16.28 lakh high-risk groups have been covered through 1,540 targeted interventions (TI) and 153 Link Worker Schemes in the country (Figure 2).
Figure 2. Number of HRG population covered by TIs over years, 2017 to 2023 (till Sep)
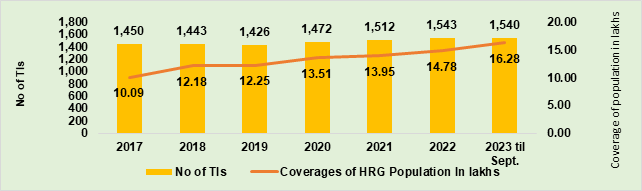
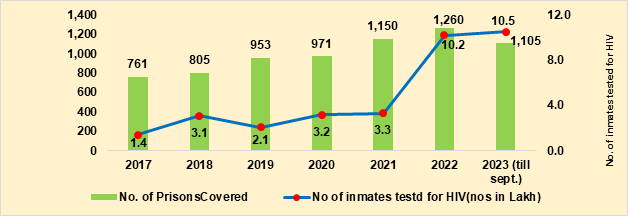
- NACP phase-V calls for the universalization of the NACP interventions in prisons and other closed settings (OCS) through a mix of service delivery models. HIV testing coverage among inmates in prisons has increased from 1.4 lakh in 2017 to 10.2 lakh in 2022 (Figure 3). During 2023, NACP interventions offering both HIV and TB services reached 1,527 institutions which includes 1,105 prisons and 422 OCS covering more than 10.8 lakh inmates and identifying 5,463 People Living with HIV/AIDS (PLHIV). Most (91%) were put on treatment. Further, 797 TB cases were identified and 788 (99%) were put on DOTS.
Figure 3. Number of Prisons and inmates covered over years, 2017 to 2023 (till Sep)
- Sampoorna Suraksha Kendras (SSK) has been set up to provide services through a single window model for the HIV-negative ‘at-risk’ population across the prevention-test-treat-care continuum to keep them HIV-free. In 2022, the Sampoorna Suraksha Model has been piloted at 10 centres in three States of the country. In the second phase, as on September 2023, the programme has expanded the SSKs to 150 centres across the 20 States of the country.
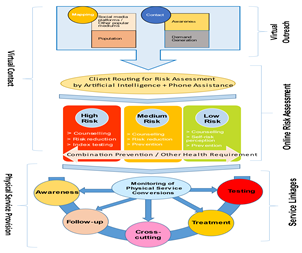
- NACP phase-V recognizes the need to reach out to newer population groups to further expand the coverage of interventions, with a specific focus on the prevention of new HIV infections. Accordingly, it calls to develop and scale up sustainable models for the ‘at-risk’ virtual population. NACO has already released a White Paper on Virtual Interventions outlining the framework for virtual interventions with a specific focus on ethics, confidentiality and data security while indicating a potential mechanism for virtual outreach and service packages based on an extensive literature search (Figure 4). Based on the White Paper, various models of interventions on virtual platforms are being tested to further inform the programme.
Figure 4. Contours of virtual interventions
C. Reducing AIDS-related mortality
Reducing AIDS-related mortality by 80% is one of the five high-level goals of NACP phase-V. This would require 95% of HIV-infected people to know their HIV status, 90% to be on ART and 86% to be virally suppressed. NACP-V aims for accessible, affordable, and quality testing and treatment services as one of the building blocks for the attainment of reductions in AIDS-related mortality.
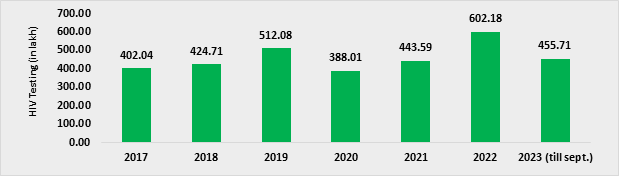
- In 2022, around 6.02 Crore HIV tests were done under NACP (Figure 5). This included HIV testing of around 2.27 Crore pregnant women. During 2023, till September, approximately 4.56 Crore HIV tests have been undertaken in the programme through various models (Figure 5).
Figure 5. HIV testing (in lakh) over years, 2017 to 2023 (till Sep)
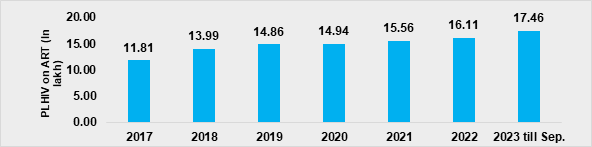
- As on September 2023, around 17.46 lakh PLHIV are on anti-retroviral (ARV) treatment in the country, including approximately around 1.06 lakh PLHIV taking ARV from private sector (Figure 6). This is around 1.35 lakh more than the on-ART PLHIV in December 2022. Transitions of PLHIV on high-quality lifelong free Dolutegravir-based regimen, which fast-tracks viral load suppression and significantly improves the quality of life, has been a key achievement under the programme.
Figure 6. PLHIV on-ARV over years, 2017 to 2023 (as on Sep)
- To monitor and improve the quality of care for HIV-infected people, the Government of India initiated free routine viral load test for PLHIV in February 2018. The number of viral load tests under NACP is continuously increasing. In 2018, around 2.40 lakh viral load tests were done. In 2022, around 10.84 lakh viral load tests were done which is almost 4 times more than the tests done in 2018 (Figure 7). During 2023, till September, around 8.74 lakh viral load testing has been done completely through the laboratory networks under the Government Health System.
Figure 7. Routine viral load testing over years, 2017 to 2023 (till Sep)
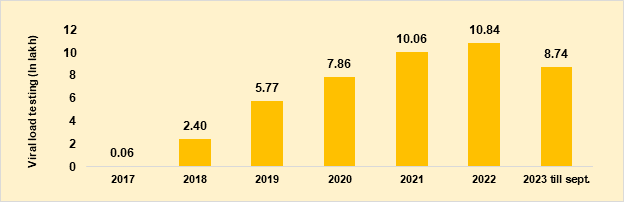
Quality laboratory services are critical enablers to the national AIDS and STD response across the prevention-detection-treatment cascade. The quality of laboratory services under NACP continues to be high. In 2022-23, among the laboratories and HIV counselling and testing centres participating in the quality assurance system, the discordance results were less than 0.05%. The six laboratories designated for early infant diagnosis scored a perfect 100% in the panel testing with no discordance results.
- The outcome of quality testing and treatment services being offered under the programme has been significant. As on September 2023, almost 20.05 lakh PLHIV are aware of their HIV status in comparison to the 16.99 lakh PLHIV in March 2019. The number of PLHIV on-ART increased from 13.99 lakh in March 2019 to 17.46 lakh in September 2023. The proportion of PLHIV who were on-ART and virally suppressed increased from 72% in 2018-19 to 93% in 2023-24.
D. Elimination of vertical transmission of HIV and syphilis
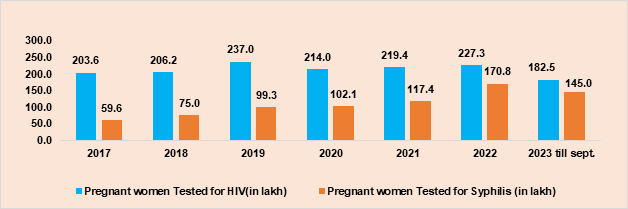
NACP phase-V calls for the attainment of the elimination of vertical transmission of HIV and Syphilis as one of the five top-level goals. This would require 95% of pregnant women to be aware of their HIV and Syphilis Status. During 2023, till September, almost 1.83 crore pregnant women have been already tested and around 1.45 crore Syphilis tests were done among pregnant women in the same period (Figure 8).
Figure 8. HIV and Syphilis testing over years, 2017 to 2023 (till Sep)
- NACP is further fine-tuning its strategic roadmap to fast-track the progress on the elimination of vertical transmission. The strategic roadmap is being piloted in seven high-priority States. The learning from the pilot will inform the finalization of operations in line with the NACP phase-V goals.
E. Universal access to quality management of sexually transmitted infections (STI)/ reproductive tract infections (RTI) services
- Sexually Transmitted Infections are indicative of ongoing unprotected high-risk sexual intercourse. For areas where HIV infection is not well established, the high prevalence of STI is an early warning of the epidemic potential of HIV from sexual transmission. Besides, one of the STIs, Syphilis is of particular concern for maternal and child health given the commitment toward the elimination of congenital syphilis.
- Given the associations, the management of STI/RTI has been one of the focus areas under NACP since its first phase. NACP phase-V has reinforced the focus on STI management by including it as one of the five high-level goals. Goal 4 of the NACP phase-V calls for the promotion of universal access to quality STI/RTI services to at-risk and vulnerable populations through a ten-pronged strategy.
- In the first six months of the third year of implementation, around 50.70 lakh clients were managed for STI/RTI episodes under the programme. NACO is leveraging improved laboratory technologies to enable equal access to integrated services for HIV and syphilis. NACP phase- V has adopted the rapid dual test kit for HIV & Syphilis, with a specific framework on follow-up testing and treatment algorithms, increasing testing uptake in a very cost-efficient manner.
F. Elimination of HIV/AIDS-related stigma and discrimination
Building upon the gamechanger initiatives and in line with Government commitment to ‘Sabka Saath, Sabka Vikas, Sabka Vishwas, Sabka Prayaas’, NACP phase-V calls for the elimination of HIV/AIDS-related stigma and discrimination as one of the five top-level goals.
- The strategies adopted under the NACP have always kept the HRG and PLHIV at the centre of its response. With the notification of the HIV/AIDS (Prevention and Control) Act 2017 and the decriminalization of section 377 of the Indian Penal Code, the country has brought significant structural changes to eliminate HIV/AIDS-related stigma and discrimination.
- The Government of India has notified the HIV and AIDS Policy for Establishments 2022 which provides a framework to mitigate issues surrounding HIV and AIDS in a workplace setting and encourages action on the part of the employer, employee, and establishments to eliminate HIV-related stigma and discrimination. Additionally, nine Central Government Guidelines under the HIV and AIDS (Prevention & Control) Act, 2017 have also been notified. Twenty-seven States/UT has appointed an ombudsman at the State level who is responsible for handling cases of HIV-related stigma and discrimination.
G. Newer initiatives
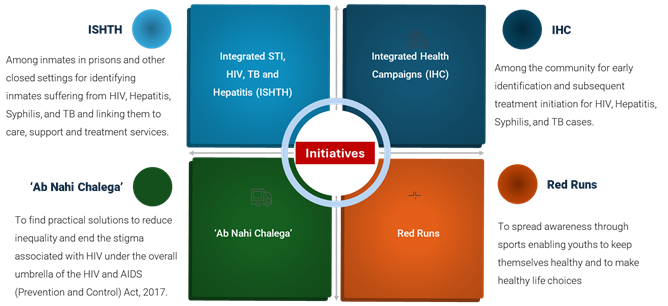
- To fast-track the progress on the targets set under phase V, several initiatives have been taken under NACP in 2023 (Figure 9). The integrated campaigns (ISHTH: Integrated STI, HIV, TB and Hepatitis) among inmates in prisons and other closed settings have been conducted in 3600 camps and identified over 1500 HIV-positive cases, 2700 Hepatitis B-positive and around 8700 Hepatitis C-positive inmates. The community-based integrated health campaigns (IHC) for HIV, Syphilis, TB, and Hepatitis in the northeastern region, especially in the States of Assam, Arunachal Pradesh, and Tripura, have been hugely successful. As a result of these campaigns, the progress on the proportion of PLHIV who are aware of their HIV status has increased from 58% in March 2023 to 67% in October 2023 in these three States, which is the highest jump since we started to track the progress on 95-95-95 in 2017.
- Following the spirit of healthy lifestyle and wellness among the young population, the first-ever RED RUNs were organized by SACSs. The objective of the RED RUN was to spread awareness through sports which can help the youth to keep themselves healthy and enable them to make healthy life choices. Winners of the State level RED RUNs participated in the ‘National RED RUN Finale’ (10 km run) in Goa in October 2023. The final event was a huge success and was attended by the Hon’ble Chief Minister of Goa, the Hon’ble Health Minister of Goa, other important dignitaries, communities, the public, winners of the State Level RED RUNs and officials of NACO and all participating State AIDS Control Societies.
- Social media strategy of NACO has been revamped and it has played a vital role in reaching out to a larger group of audience specially the youth in a highly strategized and cost-effective manner. Since last year, these focused campaigns has reached out to more than 13 million digital audiences. The ‘Ab Nahi Chalega’ campaign is nation’s efforts to focus on finding practical solutions to reduce inequality and end the stigma associated with HIV under the overall umbrella of the HIV and AIDS (Prevention and Control) Act, 2017.
Figure 9. Newer initiatives under NACP in 2023
H. Impact
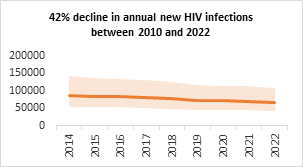
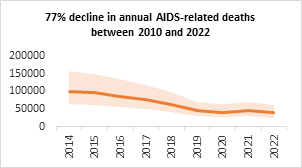
- As a result of strong political leadership, evidence-led policies and strategic implementations, the national AIDS response has been highly successful. As per the latest HIV Estimates of the Government of India, HIV prevalence in India continues to be low with an adult prevalence of 0.20% in 2022. The annual new HIV infections have declined by 42% between 2010 and 2022, against the global average of 38%; and the AIDS-related mortalities have declined by 77%, against the global average of 51%.
Figure 10. Trend of annual new HIV infection and annual AIDS related death
|
|
|
- Maternal Health
As per the Special Bulletin on MMR released by the Registrar General of India (RGI), the Maternal Mortality Ratio (MMR) of India has improved further by a spectacular 6 points and now stands at 97/ lakh live births. The Maternal Mortality Ratio (MMR) is defined as the number of maternal deaths during a given time periodper100,000 live births.
As per the statistics derived from Sample Registration System (SRS), the country has witnessed a progressive reduction in MMR from 130 in 2014-2016, 122 in 2015-17, 113 in 2016-18, 103 in 2017-19 and to 97 in 2018-20.Upon achieving this, India has accomplished the National Health Policy (NHP) target forMMR of less than 100/lakh live births and is on the right track to achieve the SDG target of MMR less than70/lakh live births by 2030.
The outstanding progress made in terms of the number of states which have achieved Sustainable Development Goal (SDG) target, the number has now risen from six to eight leading with Kerala (19), followed by Maharashtra (33), then Telangana (43) and Andhra Pradesh (45), subsequently Tamil Nadu (54), Jharkhand(56), Gujarat(57) and lastly Karnataka (69).
-
-
- Key highlights of NFHS-5(2019-21)-Maternal Health:
-
- 1st Trimester ANC Registration increased from 58.6% (NFHS-4) to 70% inNFHS-5
- Institutional Deliveries increased from 78.9% (NFHS-4) to 88.6% in NFHS-5
- Skilled Birth Attendant (SBA) attended deliveries increased from 81.4%(NFHS-4) to 89.4%inNFHS-5.
-
-
- Surakshit Matritva Aashwasan (SUMAN): It aims to provide assured, dignified, respectful and quality healthcare at no cost and zero tolerance for denial of services for every woman and newborn visiting the public health facility to end all preventable maternal and newborn deaths. Till 15th December 2023, 38,096 facilities have been notified under SUMAN.
- Maternal Perinatal Child Death Surveillance Response (MPCDSR) software was launched by the Hon’ble Union health Minister of Health & Family Welfare in September 2021. This was followed by the National ToT of the software in October2021. Maternal Perinatal Child Death Surveillance Review (MPCDSR) software has been roll out in all States/UTs since 2021-22.
- Midwifery Educator Training: The Government of India has taken a policy decision to roll out Midwifery Services in the country in order to improve the quality of care and ensure respectful care to pregnant women and newborns. “A guideline on Midwifery Services in India, 2018” was released during the Partners Forum held in December 2018 at New Delhi.
-
- Resumption of Midwifery training: Training of Midwifery Educators (MEs) was halted due to the pandemic, which was resumed in September 2021 at NMTI in Telangana.
- Release of Scope of Practice: “Scope of Practice document for Mid wifery Educators (ME) and Nurse Practitioner Midwife (NPM)” has been released in collaboration with the Indian Nursing Council (INC).It acts as a guiding document for their education, regulation and ongoing professional development.
-
- Pradhan Mantri Surakshit Matritva Abhiyan (PMSMA): Since inception, more than 4.61crore antenatal check-ups have been conducted and 49.56 lakh high risk pregnancies have been identified under PMSMA across States/ UTs till 15th December 2023.
- LaQshya: It aims to improve the quality of care in Labour Room and Maternity Operation Theatres to ensure that pregnant women receive respectful and quality care during delivery and immediate postpartum. Till 30th November 2023, 873 Labour Rooms and 663 Maternity Operation Theatres are LaQshya certified at national level. During the FY2022-23, 185 Labour Rooms and 129 Maternity Operation Theatres are LaQshya certified at national level.
- Janani Suraksha Yojana (JSY):JSY is a safe motherhood intervention under the National Health Mission (NRHM). Launched with the objective of reducing maternal and neonatal mortality, the Janani Suraksha Yojana (JSY) promotes institutional delivery among pregnant women especially with weak socio-economic status i.e. women from Scheduled Castes, Scheduled Tribes and BPL households.
-
Under JSY 43.35 lakhs beneficiaries received benefits during the period of April-September 2023 (Provisional data, FY 2023-24).
-
-
- Guideline released:
-
- In continuation to PMSMA initiative, Extended PMSMA strategy was launched in January 2022 to ensure quality ANC to pregnant women, especially to high-risk pregnancy (HRP) women and individual HRP tracking till a safe delivery is achieved by means of financial incentivization for the identified high risk pregnant women and accompanying ASHA for extra 3 visits over and above the PMSMA visit.
- Optimizing Postnatal Care guideline was launched in June 2023 which aims to strengthen the quality of post-natal care by laying emphasis on detection of danger signs in mothers and Incentivization of ASHAs for prompt detection, referral & treatment of such high risk postpartum mothers.
Child Health
-
-
- As per the latest report of Sample Registration System (SRS) released in October 2021 by the Registrar General of India (RGI), Infant Mortality Ratio (IMR) of India has declined from 32 per 1000 live births for the year 2018 to 30 per 1000 live births for the year 2019. 27 States/ UTs namely Mizoram, Nagaland, Sikkim, Kerala, A & N Islands, Goa, Lakshadweep, Puducherry, Manipur, Delhi, D & N Haveli, Chandigarh, Tamil Nadu, Maharashtra, Daman & Diu, Punjab, Himachal Pradesh, Jammu & Kashmir including Ladakh, West Bengal, Karnataka, Tripura, Telangana, Andhra Pradesh, Gujarat, Haryana, Jharkhand, Uttarakhand have achieved National Health Policy Target (28 per 1000 live births by 2019).
-
-
-
- Facility Based Newborn Care (FBNC) program: 1054 Special Newborn Care Units (SNCUs) at District/ Medical College Level and – 2,774 Newborn Stabilization Units (NBSUs) at the level of FRUs/ CHC levels are functional to provide services to sick and small newborns. A total of 9.85 lakhs newborns received treatment in Special Newborn Care Units (SNCUs) at District Hospitals and Medical Colleges (April-November, 2023).
- National Newborn Week is observed from 15th to 21st November every year to reinforce the importance of newborn health as a key priority area and reiterates its commitment at the highest level. In the year 2023 also, a National Workshop for the National Newborn Week was organized by MoHFW on 17th November 2023. The theme of National Newborn Week for this year is “Nurturing Newborn Lives through Community – Facility Engagement”. National Newborn Week and SAANS Campaign IEC posters were also released by MoHFW on this day for dissemination of information and for triggering behaviour change and demand generation on newborn health along with the release of various Child Health Guidelines (Integrated Management of Neonatal and Childhood Illness, Facility Based Newborn Care & Pediatric Centre of Excellence)
-
-
-
- MusQan – Quality improvement initiative of Child Health services: The Hon’ble Union Minister of Health and Family Welfare launched “MusQan” initiative on 17th September 2021 for ensuring child friendly services in Public Health facilities on the occasion of World Patient Safety Day. The initiative will be focusing on improving the quality parameters for ensuring safety and availability of infrastructure, equipment, supplies, skilled human resources, clinical protocols, evidence based practices etc. at public health facilities. As on November 2023, total 58 facilities got national level certification under MusQan.
-
- Home Based Newborn Care (HBNC) program: A total of 1.47 crore newborns received complete schedules of home visits by ASHAs whereas more than 8 lakhs identified sick newborns were referred to health facilities by ASHAs during the period of 2022-23. F.Y. 2023-24 (Qtr.-1), a total of 33.5 lakhs newborns were completed with scheduled visits by ASHA and out of which 1.95 lakhs newborns are identified as sick and referred to health facility under HBNC programme.
b). Home Based Care of Young Child (HBYC): In FY 2022-23, approval has been accorded for 690 Districts including all Aspirational Districts to implement HBYC across States/UTs except Goa. More than 2.5 crores home visits conducted to young children (3 months-15 months) by ASHAs during the year 2022-23. F.Y. 2023-24 (Qtr.-1) more than 81 lakhs home visits were conducted to children (3-15 months) by ASHA under HBYC Programme. Further, in order to focus on quality home visit to children under HBNC & HBYC programme with a on job hand holding support to ASHAs, a supportive supervision handbook for ASHA Facilitators and ANM/MPW on HBNC and HBYC programs has been provided followed by national orientation with all States/UTs.
m) Under Intensified Diarrhoea Control Fortnight (IDCF), 2023, approximately 11 crore (provisional) children up to five years of age were provided with ORS and Zinc against the target of 13.37 crore children of the same age group. The data compilation for the IDCF/Diarrhoea prevention activities for the year 2023 round is in process.
- Nutrition
- Mothers’ Absolute Affection (MAA) to improve breastfeeding coverage which includes early initiation of breastfeeding and exclusive breastfeeding for first six months followed by age-appropriate complementary feeding practices through capacity building of frontline health workers and comprehensive IEC campaigns. As per National Family Health Survey-5 (NFHS-5), the rates of Early Initiation of Breastfeeding, Exclusive Breastfeeding for six months, and timely introduction of complementary foods at 6-8 Months are 41.8 per cent, 63.7 per cent and 45.9 per cent respectively.
- National Deworming Day (NDD): Under NDD, albendazole tablets are administered in a single fixed day approach via schools and anganwadi centres in two rounds (February and August) to reduce the soil transmitted helminth (STH) infestation among all children and adolescents (1-19 years). For NDD 2023 February round, 24.21 crore children in the age group of 1-19 years were provided Albendazole tablets against the target of 27.43 crore children of the same age group.
- Nutrition Rehabilitation Centres (NRCs): There are 1129 Nutrition Rehabilitation Centres (NRCs) operational across the country in 29 States/UTs. In FY 2023-24 (April – June 2023), 0.56 Lakhs children suffering from Severe Acute Malnutrition (SAM) with medical complications were admitted and received treatment in NRCs. .
- Lactation Management Centres (LMCs): As of FY 2023-24 (April – June 2023) 53 Comprehensive Lactation Management Centres (CLMCs) and 65 Lactation Management Units (LMUs) are supported under NHM
Anemia Mukt Bharat (AMB) programme
The progress for the FY 2023-24 (April – September 2023) is as follows:
- 3.7 Crore children of age group 6-59 months were provided Iron and Folic Acid (IFA) syrup every month
- 4.2 Crore children of age group 5-9 years were provided IFA Pink tablets every month
- 5.2 Crore children of age group 10-19 years were provided IFA Blue tablets every month
- 1.5 Crore pregnant women and 80 lakh lactating women were provided 180 IFA Red tablets during antenatal Care and Postnatal Care period respectively.
- Rashtriya Bal Swasthya Karyakram (RBSK): As reported by States/UTs in HMIS during April-November, 2023,11.62 crores children have been screened by Mobile Health Teams. 41.26 Lakh newborn have been screened at Delivery points under RBSK Program during April-November, 2023.
- Adolescent Friendly Health Clinics (AFHCs): Adolescent Friendly Health Clinics (AFHCs) act as the first level of contact of primary health care services with adolescents. The primary aim is provision of counselling and clinical services to the visiting adolescent client. 64.8 lakh adolescents registered at Adolescent Friendly Health Clinics (AFHCs) in FY 2023-24 till second quarter.
- Weekly Iron Folic Acid Supplementation (WIFS) entails provision of weekly supervised IFA tablets to in-school boys and girls and out-of-school girls for prevention of iron and folic acid deficiency. 5.8 crores adolescents had been provided Weekly Iron Folic Acid Supplementation (WIFS) every month besides Nutrition Health Education in FY 2023-24 till second quarter.
- Scheme for Promotion of Menstrual Hygiene among Adolescent Girls: In the age group of 10-19 years with specific reference to ensuring health for adolescent girls. The scheme aims to ensure that adolescent girls have adequate knowledge and information about menstrual hygiene, use of sanitary napkins and environmentally safe disposal mechanism. It also aims to ensure that high- quality and safe products are made available to them. Around 32.1 lakh adolescent girls were provided sanitary napkins every month in FY 2023-24 till second quarter.
- Peer Educator program: aims to ensure that adolescents are benefitted from regular and sustained peer education covering nutrition, sexual and reproductive health, conditions for non-communicable diseases (NCDs), substance misuse, injuries and violence (including gender-based violence) and mental health. Total 2.4 Lakh PEs were selected and a 1.3 lakh Adolescent Health and Wellness Days (AHWDs) were held during FY 2023-22 till second quarter.
Ayushman Bharat School Health and Wellness:
- School Health & Wellness Programme (launched in February 2020) is being implemented in government and government aided schools in Districts (including most of the Aspirational Districts) of the country in the first phase of the implementation.
- Two teachers, preferably one male and one female, in every school, designated as “Health and Wellness Ambassadors” (HWAs) shall be trained to transact health promotion and disease prevention information on 11 thematic areas in the form of interesting joyful activities for one hour every week. School Health & Wellness Programme (SH&WP) reached to 388 districts in 34 States/ UTs. Around 6.14 lakh Health and wellness Ambassadors (HWAs) trained up to September ’23.
Social Awareness and Actions to Neutralize Pneumonia Successfully (SAANS): SAANS Campaign has been rolled-out in the States/ UTs from 12th November, 2023 – 29th February 2024 with the aim to accelerate the action against Childhood Pneumonia by generating awareness around protect, prevent and treatment aspects of Childhood Pneumonia and to enhance early identification and care seeking behaviours among parents and caregivers. Additionally, awareness generation, promotion and administration of Pneumococcal Vaccine (PCV) has also been included under SAANS campaign for the year 2021.
- Family Planning
Key highlights of NFHS-5 (2019-21):
o In NFHS-5(2019-21) 32 States/UTs have shown reduction in early marriages and 25 have shown reduction in prevalence of teenage pregnancies as compared to NFHS-4.
o NFHS-5 (2019-21) has reflected those women aged 15-24 yrs who use hygienic methods of protection during their menstrual period have increased to 77.3% from 57.6% (NFHS-4). 35 out of 36 States/ UTs have shown significant improvement in use of hygienic methods during menstruation.
- Total Fertility Rate (TFR) has declined from 2.7 in NFHS 3 (2005-06) to 2.0 in NFHS 5 (2019-21) which is below replacement level.
- Out of 36 States/UTs, 31 States/UTs have achieved replacement TFR of 2.1 or less.
- Modern Contraceptive usage has increased substantially from 48.5% from NFHS 3(2005-06) to 56.5% in NFHS 5 (2019-21).
- Unmet Need for Family Planning has declined from 12.8% in NFHS 3 (2005-06) to 9.4% in NFHS 5(2019-21)
- NFHS 5 shows an overall positive shift towards spacing methods (increase in all spacing methods).
The performance of Family Planning services in FY 2023-24,up to Nov 2023
- Total Sterilization: 13.05 Lakhs
- Post-partum IUCD (PPIUCD): 21.90 Lakhs
- PPIUCD acceptance rate (%) in public health facilities: 27.7 %.
- Contraceptive Injectable MPA (Antara Program): 26.42 lakh doses have been administered
- Centchroman (Chhaya): 75.79 Crore strips of Centchroman (Chhaya) have been distributed.
Mission Parivar Vikas:
The Government launched Mission Parivar Vikas (MPV) in 2016 for substantially increasing access to family planning services in 146 high fertility Districts with Total Fertility Rate (TFR) of 3 and above in seven high focus States (Uttar Pradesh, Bihar, Rajasthan, Madhya Pradesh, Chhattisgarh, Jharkhand and Assam). In November 2021, the Scheme was extended to remaining districts of the seven high focus States and all districts of six North Eastern States (Arunachal Pradesh, Manipur, Meghalaya, Tripura, Nagaland and Mizoram), where the modern contraceptive usage is low and unmet need for Family Planning is high.
The performance Family Planning services of in MPV States in FY 2023-24 (Up to Nov)
- Total number of Sterilizations: 4.23 lakh sterilization
- Post-partum IUCD (PPIUCD): 12.62 lakh PPIUCD
- PPIUCD acceptance rate (%) in public health facilities: 15.3 %
- Contraceptive Injectable MPA (Antara Program): 16.69 lakh doses
- Centchroman(Chhaya):51.43 lakh Strips of Centchroman (Chhaya) have been distributed.
- Pre-Conception and Pre-Natal Diagnostic Techniques (PC & PNDT):
- As per Quarterly Progress Report (QPR) of June 2023, submitted by the States/UTs, total 82,281 bodies have been registered under the PC& PNDT Act. So far, a total of 4,853 machines have been sealed and seized for the violations of the law. A total of 3,563 court cases have been filed under the Act and 731 convictions have so far been secured, leading to suspension/cancellation of medical licenses of 145 doctors.NFHS-5 (2019-21) has also recorded improvement of 10 points in the sex ratio at birth at the national level from 919 in NFHS-4 to 929. 23 States/UTs have shown improvement whereas 13 States/UTs show decline in sex ratio at birth.
- A two day national capacity building workshop was conducted for State/UT Appropriate authorities and State/UT PNDT nodal officers on 5th & 6th July 2023 followed by one day Training of Trainers (TOT) on 8th July, 2023 for State/UT trainers.
- National review meeting was held with all State/ UT health ministers under the chairpersonship of HFM in Swasthya Chintan Shivir held on 14-15th July 2023 at Dehrandun.
- 29th meeting of Central Supervisory Board (CSB) under PC & PNDT Act, 1994 was held on 18 October, 2023under the chairpersonship of Hon’ble Union Health Minister on virtual platform.
- Review meetings were conducted in all 36 States/UTs and implementation of PC&PNDT Act was reviewed in all aspects.
- Capacity building of District Appropriate Authorities and PNDT Nodal Officers was conducted in the Delhi.
- Mera Aspataal:
The government has launched the “Mera Aspataal/My Hospital” initiative to empower the patients by seeking their views on Quality of experience in a public healthcare facility. Mera Aspataal/My Hospital is a simple, and multi-lingual application that captures patient feedback in a very short time on the services received from public hospitals. It works through multiple communication channels, including Short Message Service (SMS), Outbound Dialling (OBD), a mobile application, and a web portal. The application allows feedback to be consolidated, analysed, and disseminated on a frequently updated dashboard. Analysed data is used to improve the quality of services in healthcare facilities. Thus, Mera Aspataal allows patients to connect with the healthcare providers and policymakers and to have their opinions heard and acted upon.
- Kayakalp:
The government has launched the “Mera Aspataal/My Hospital” initiative to empower the patients by seeking their views on Quality of experience in a public healthcare facility. Mera Aspataal/My Hospital is a simple, and multi-lingual application that captures patient feedback in a very short time on the services received from public hospitals. It works through multiple communication channels, including Short Message Service (SMS), Outbound Dialling (OBD), a mobile application, and a web portal. The application allows feedback to be consolidated, analysed, and disseminated on a frequently updated dashboard. Analysed data is used to improve the quality of services in healthcare facilities. Thus, Mera Aspataal allows patients to connect with the healthcare providers and policymakers and to have their opinions heard and acted upon. At present 11,034 health facilities in 34 states and UTs are integrated with “Mera Aspataal’.
- eHealth inputs for Budget Speech
A. Ayushman Bharat Digital Mission-ABDM:
The Ayushman Bharat Digital Mission-ABDM (earlier known as National Digital Health Mission) was launched with a vision to create a national digital health ecosystem that supports universal health coverage in an efficient, accessible, inclusive, affordable, timely, and safe manner.
ABDM aims to enable the creation of longitudinal electronic health records across the health spectrum for citizens, make healthcare accessible for citizens, reduce the cost of care and enable greater efficiencies in health service delivery. The digital health ecosystem created by ABDM supports continuity of care across primary, secondary, and tertiary healthcare in a seamless manner. It ensures availability of health care services through electronic means particularly in remote and rural areas where generally as such specialist care may not be available.
Achievement of ABDM: As on 19th December, 2023,
(i) Ayushman Bharat health accounts created: 49.86 crore
(ii) Healthcare professionals are registered under Ayushman Bharat Digital Mission: 2,58,217
(iii) Health facilities registered under ABDM: 2,25,968
B. National Telemedicine Service-eSanjeevani
National Telemedicine Service-eSanjeevani provides access to specialized medical healthcare across the country by providing facility for doctor-to- doctor consultation and patient-to-doctor consultation. This initiative aimed to make healthcare services more accessible, especially in rural and remote areas. It enables people, including those in rural and underserved areas, to consult with healthcare professionals without the need for physical travel to healthcare facilities. This initiative has also been instrumental in expanding healthcare services to a broader population, reducing the gap in access to medical care, and leveraging digital technology to provide healthcare services where they are needed most.
Achievement of eSanjeevani:
(i) eSanjeevani consultations rendered: more than 18.9 crore
(ii) it is operational in more than 1,33,000 Health & Wellness Centres and 27,000 hubs
C. Global Initiative on Digital Health:
Under India’s G 20 Presidency, India identified Digital Health as one of its key health priorities namely- “Digital Health Innovation & Solutions to aid UHC & improve Healthcare Service Delivery” and proposed the Global Initiative on Digital Health – a WHO Managed Network as an institutional framework for the development of global digital health ecosystem. The GIDH was successfully launched on 19th August, 2023 during the G20 Health Minister Meeting. It intends to create a ‘common platform’ to converge global efforts for digital health and bridge the digital divide by promoting equitable access to technological tools.
- Medical Education
a) The historic National Medical Commission Act was passed by the Parliament in August, 2019. Now, the National Medical Commission has been constituted with effect from 25th September, 2020 and the years old MCI has been dissolved and the Indian Medical Council Act, 1956 has been repealed. The principal change in the regulatory mechanism is that the regulator will be primarily ‘selected’ rather than ‘elected’. The National Medical Commission will steer the reforms in medical education. This will include increase in UG & PG seats along with improved access to quality and affordable medical education and maintaining high ethical standards in medical profession, implementation of National Exit Test (NEXT) as per NMC Act, 2019 for the medical graduates.
b) Considering high fees structure, NMC Act empowered the Commission to frame guidelines for determination of fees and all other charges in respect of 50 percent of seats in private medical colleges and deemed to be Universities, to make medical education affordable. Accordingly, the NMC has released guidelines for mandatorily capping tuition fees for 50% MBBS and postgraduate medical seats in private medical colleges and deemed to be universities.
c) There is an increase of 82% in medical colleges from 387 before 2014 to 706 (Govt.: 389, Pvt.: 317) as of now. Further, there is an increase of 112% in MBBS seats from 51,348 before 2014 to 1,08,940 as of now, there is also an increase of 127% in PG seats from 31,185 before 2014 to 70,674 as of now.
d) Under the Central Sponsored Scheme for establishment of new medical colleges, establishment of 157 medical colleges have been approved in three phases, of which 108 are functional and remaining will be functional in a few years. Of these 157 colleges, 40 are coming up in the Aspirational Districts of the country thereby addressing the issues of inequity in medical education.
e) Rationalization of Minimum Standards Requirements (MSR): The MSRs for establishment of medical colleges have been streamlined. This will reduce the cost of establishment of new medical colleges and increase intake capacity.
f) Two years post MBBS Diplomas by National Board of Examinations: Keeping in view the importance of Diploma courses to meet the shortfall of postgraduate students and augment healthcare in remote parts of the country, the National Board of Examinations (NBE) has launched diplomas in eight disciplines namely – Anaesthesia, Gynaecology & Obstetrics, Pediatrics, ENT, Ophthalmology, Family Medicine, Tuberculosis & Chest Diseases and Medical Radiodiagnosis.
g) District Residency Programme (DRP) has been implemented for the students in the third or fourth or fifth semester of Post Graduate Programme. Colleges may apply for proportionate increase of seats, keeping in view that one resident has to be away for 3 months out of total 36 months of training, which means that proportionate increase shall be increase of 1 seat against 12 existing seats (3 months divided by 36), after one year of implementation of DRP.
- National Programme for Tobacco Control and Drug Addiction Treatment [NPTCDAT]
- Regulation of depiction of tobacco usage on Over-The-To-Media (OTT) platforms: To regulate tobacco use depiction on Over-The-Top (OTT) media platforms, Government of India has notified Cigarettes and other Tobacco Products (Prohibition of Advertisement and Regulation of Trade and Commerce, Production, Supply and Distribution) [COTP] Amendment Rules, 2023 vide GSR No. 400 (E) dated 31st May, 2023 . As per the COTP (Amendment) Rules, 2023, every publisher of online curated contents displaying tobacco products or their use will comply with (a) display anti-tobacco health spots, of minimum thirty seconds duration each at the beginning and middle of the programme; (b) display anti-tobacco health warning as a prominent static message at the bottom of the screen during the period of display of the tobacco products or their use in the programme; (c) display an audio-visual disclaimer on the ill-effects of tobacco use, of minimum twenty seconds duration each, in the beginning and middle of the programme.
- Global commitments: India has been pioneering as the global leader in tobacco control measures. Ministry of Health & Family Welfare, Government of India hosted the Seventh Meeting of the WHO’s Global Tobacco Regulators’ Forum (GTRF) from 25-27 April, 2023. The meeting led to discussions regarding the regulation of novel and emerging Nicotine and Tobacco products, as well as conventional products; and develop specific action points for GTRF members to continue sharing information, through current and newly identified intersessional priorities.
- Online Reporting Mechanism: To streamline the quarterly reporting of National Tobacco Control Programme (NTCP) activities a Dashboard of the NTCP MIS was launched on 31st May, 2023 by Hon’ble Union Minister for Health & Family Welfare. This dashboard reflects real-time data on various NTCP activities reported / uploaded by States/UTs .
- Portal for reporting online violations: Portal (https://violation-reporting.in/) to report Online violations under COTPA, 2003 and prohibition of e-cigarette Act, 2019 was launched to strengthen the enforcement of these Acts. On receipt of the online violation, takedown notices will be served to the intermediaries to remove the unlawful content (as per IT Rules, 2021).
- Tobacco Free Youth Campaign: Tobacco Free Youth Campaign was launched across all States and Union Territories to create intensive awareness on harmful effects of tobacco use, particularly amongst the youth. The States took forward these 60 days campaign from 31st May to 31st July 2023 for effective enforcement of tobacco control laws; implementation of guidelines for Tobacco Free Educational Institutions; Tobacco Free Villages and IEC strategies. The best performing States/UTs were felicitated with a trophy and a certificate in a National Review Meeting held on 20th October, 2023 to the Govt. of Karnataka (Best performer for Monitoring Tobacco control laws), Govt. of Rajasthan (Award for Excellence in Tobacco Control activities), Govt. of Himachal Pradesh (Best performer for creating awareness), Govt. of Punjab (Best performer for ToFEI implementation amongst the big State), Govt. of Odisha (Best performer for Tobacco Free Village initiative) and UT Administration of Dadra and Nagar Haveli & Daman and Diu (Best performer for ToFEI implementation amongst the smaller State). The Awards were received by respective State Nodal Officer of the State for Tobacco Control.
- Implementation of Prohibition of e-Cigarettes Act, 2019 (PECA, 2019): Effective steps have been undertaken for strengthening the implementation of “The Prohibition of Electronic Cigarettes (Production, Manufacture, Import, Export, Transport, Sale, Distribution, Storage and Advertisement) Act, 2019”. Law enforcers workshop was organized in February, 2023 in collaboration with National Law School of India University (NLSIU), Bengaluru to plan concrete steps for effective compliance of the prohibition of such novel products. A Public Notice was published PAN India for effective compliance of the PECA, 2019. A meeting of experts was also convened under DGHS in November 2023 to update the scientific evidence with meta-analysis on ENDS (e-cigarettes).
- Strengthening of Tobacco Cessation Services: Tobacco cessation efforts contribute significantly to promoting good health, preventing diseases, and ensuring well-being, all of which align with Sustainable Development Goal 3’s objectives. Efforts have been taken to strengthen and scale up the tobacco cessation services in India. An expert committee to accelerate tobacco cessation services with three sub-groups focusing on the development of National Strategic Action Plan, Training and capacity building and IEC activities have been constituted. Further, another expert group constituted is working and expediting the process on the development of Operational Guidelines to establish Tobacco Cessation Centers in Medical colleges in coordination with Medical Education Division.
- North Eastern Indira Gandhi Regional Institute of Health & Medical Sciences (NEIGRIHMS), Shillong, Meghalaya.
NEIGRIHMS is a super specialty teaching Institute established in 1987 in Shillong under the Meghalaya Societies Regulation Act 1983 with an objective to provide advanced and specialized medical facilities of the highest level in selected specialties, and to serve as a regional referral service centre for comprehensive health care of people in North Eastern States. It has been designed as a Postgraduate Medical Institute in the lineage of AIIMS, New Delhi and PGIMER, Chandigarh. The Institute is under the administrative control of the Ministry of Health & Family Welfare, Government of India.
The Institute is presently having 30 fully functional Super Speciality and Speciality departments. It is offering super specialty services in Cardiology, CTVS, Neurology, Neurosurgery, Surgical Oncology and Urology, besides specialty services in General Surgery, General Medicine, Paediatrics, Obstetrics & Gynaecology, ENT, Orthopaedics, Dentistry, Psychiatry, Radiotherapy, TB & Respiratory Diseases, Dermatology and Ophthalmology. These departments are very well supported by the departments of Radiology, Anaesthesiology, Pathology, Microbiology, Forensic Medicine, Biochemistry, Anatomy, Community Medicine, Pharmacology, Hospital Administration, Transfusion Medicine & Blood Centre and Physiology. It is well equipped with all basic as well as advanced equipment’s like CT scan, 1.5 Tesla MRI, Digital Mammography system, Fully automated High Vacuum Double Door Steam Sterilizer Unit and Washer Disinfector, etc.
The hospital presently has 594 beds including 104 ICU beds with ventilators, out of which 280 beds are designated for COVID-19 including 43 ICU beds designated for COVID-19, other ICU beds includes Medical Critical Care Unit with 15 beds, Anaesthesia Critical Care Unit with 16 beds, CTVS ICU with 10 beds, ICCU with 11 beds, Paediatric ICU with 14 beds, Neonatal ICU with 6 beds each.
NEIGRIHMS is also designated as Mentor institute for the entire North Eastern States for COVID Testing facilities by ICMR, Various COVID-19 Testing facilities like RTPCR, TruNAT & CB NAAT are available in the Institute round the clock.
Academic Activities:
The Institute is conducting Post Doctoral (DM), Post-Graduate (MD/MS), Under Graduate (MBBS), M. Sc (Nursing) and B. Sc. (Nursing) courses.
MBBS Course:
The Under Graduate (MBBS) course was started in the year 2008 an annual intake of 50 MBBS students per year which will be increased to 100 annual intake. Infrastructure including college building and hostel buildings are being readied. The proposal for creation of additional Faculty and non-Faculty posts for the college has been submitted to Ministry along with proposal to increase the annual intake to 100.
Re-Distribution of Seats for UG Course
The Ministry vide letter NoU.12012/92/2007-NE dated 21/03/2023 re-distributed the seats in MBBS Course at NEIGRIHMS, Shillong as per details hereunder
|
State |
Existing % of Seat distribution |
Existing % of Seat distribution (in No.) |
New % of Seat distribution |
New Seat distribution (in No.) for a total of 50 seats |
New Seat distribution (in No.) for a total of 100 seats |
|
All India |
15 |
8 |
15 |
8 |
15 |
|
North East |
37 |
18 |
37 |
18 |
37 |
|
Meghalaya |
18 |
9 |
28.8 (60% of the Remaining 48% seats for states having no medical college) |
14 |
29 |
|
Nagaland |
15 |
8 |
19.2 (40% of the Remaining 48% seats for states having no medical college) |
10 |
19 |
|
Arunachal Pradesh |
8 |
4 |
0 |
0 |
0 |
|
Mizoram |
7 |
3 |
0 |
0 |
0 |
|
Total |
100 |
50 |
100 |
50 |
100 |
POST GRADUATE (MD/MS) COURSES:
NEIGRIHMS started the Post-Graduate (MD/MS) Courses in 2009 in 4 Departments viz: Anaesthesiology, Pathology, Microbiology and Obstetrics & Gynaecology. The seats were increase from existing 2 to 4 seats in the department of Anaesthesiology from the session 2010. The PG course in the Department of Anatomy was started from the year 2013. The PG Courses in 3 Departments viz; Radio Diagnosis & Imaging, General Surgery and General Medicine were started from the session 2013-14. Increase of seats in the Departments of Pathology and Microbiology was started from the session 2014. The Post Graduate (MD/MS) courses in the Department of Oto-Rihno Laryngology, Dermatology with annual intake of 2 seats each and MD Forensic Medicine with annual intake of 3 seats was started from the session 2019-2020. PG Courses were started from the 2020-21 in the 3 Departments viz. Biochemistry, Ophthalmology and Pharmacology with annual intake of 2 seats. PG Course in the Department of Orthopaedics has started from the session 2023-24 with annual intake of 2 seats. The Ministry has accorded approval for starting of MD Physiology and the course will be started from the next Academic Session.
Post Doctoral (DM) Courses:
Post-Doctoral (DM) Cardiology Course was started from the session 2012-13 with an annual intake of 2 seats. In addition of Post Doctoral Course in DM Neurology with an annual intake of 2 (two) seats has been started from the session 2022-23.
Nursing Education:
The B.Sc. Nursing course with 50 annual intake was started in the year 2006. The students intake will be increased to 100 seats from the next Academic session. M. Sc. Nursing Course with 10 students intake per year was started from the year 2016. Apart from the above for B.Sc Nursing course 8 (eight) seats Prime Minister’s Special Scholarship Scheme for UTs of J&K and Ladakh (PMSS) & 12 (twelve) under 25% Supernumerary seats for Foreign Students have been increased. For M. Sc Nursing Course 2 (two) seats have been increased under 25% Supernumerary seats for Foreign Students.
Academic Achievements:
- Till date 16 batches of MBBS students have been admitted and 10 batches passed out.
- Number of PG students admitted is 244 and 159 PGs in various specialties have completed their course.
- Number of DM Cardiology admitted is 24 and 16 have completed their course.
- Number of M.Sc. (Nursing) students admitted is 76 and 5 batches have completed their course.
- Number of B.Sc. (Nursing) students admitted is 900 and 12 batches have completed their course.
- National and International Conference, symposia, workshops, teleconferences and tele-medicine workshops are held in collaboration with other institutes in the country, involving various other funding agencies for research, especially ICMR, HRD, DST etc. It has been in the forefront with its research publications of appreciable impact on need-based, indigenous projects.
Management of the Institute
The Governing Council of the Institute is the highest authority of the Institute headed by the Union Health Minister as its President with 27 other Members. The Executive Committee is chaired by the Secretary, Ministry of Health &Family Welfare, Government of India. The other Committees have also been constituted such as Standing Finance Committee, Standing Selection Committee and Academic Committee, etc. The Director, NEIGRIHMS is the Chief Executive Officer of the Institute. All administrative and academic activities are under his control.
The Dean (Academics) is the overall in charge of the academic activities of the Institute and the Dean (Research) looks after the research activities. The Medical Superintendent is the overall in-charge of the hospital who looks after the day to day functioning of the hospital. The functioning of the different department is directly under the respective Heads of Department. Key areas such as Casualty, CSSD, Stores, Hospital Waste Management, Etc are looked after by designated officer under the Supervision of the Medical Superintendent.
Sanctioned Strength and Incumbency Position:
Presently the Institute is having a total of 1365 manpower in position including Faculty, Group A, B & C posts against the sanctioned strength of 2026 Posts. The Institute has been making efforts to fill up the vacant faculty posts to augment the teaching faculty in different Departments of NEIGRIHMS. The total number of faculty in position is 93 Nos. (Excluding 1 who are yet to join) against the sanctioned strength of 168 posts. The Institute till date is having 67 Nos. of Senior Residents in position out of 127 posts and 67 Junior Residents in position out of 110 posts.
Outsourced Services:
Institute has outsourced manpower services; cleaning services; security services and vehicles for additional support in its hospital clinical services, academic and research activities.
Major High End Equipments:
|
|
2021-22 |
2022-23 |
||||
|
Out Patients (OPD) |
204110 |
330482 |
||||
|
Patients registered from Casualty |
12816 |
17709 |
||||
|
IN Patients (IPD) |
10293 |
14027 |
||||
|
Death |
792 |
730 |
||||
|
Birth |
768 |
1003 |
||||
|
|
||||||
|
DETAILS OF PATIENT TREATED |
2021-22 |
2022-23 |
||||
|
OPD |
CASUALTY |
IPD |
OPD |
CASUALTY |
IPD |
|
|
Patients from North East India |
62197 |
2060 |
2717 |
115744 |
2672 |
3741 |
|
Patients from Outside North East |
1790 |
151 |
44 |
1902 |
169 |
46 |
|
Patients from Outside India |
45 |
4 |
0 |
1155 |
14 |
22 |
|
Patients from Meghalaya |
140078 |
10601 |
7532 |
211681 |
14854 |
10218 |
|
|
||||||
|
Operation Theatre |
2021-22 |
2022-23 |
||||
|
Major OT |
2361 |
3554 |
||||
|
Minor OT |
1088 |
1594 |
||||
|
|
2021-22 |
2022-23 |
||||
|
Total no. of Beds |
594 |
594 |
||||
|
|
||||||
|
Investigations & Procedures |
2021-22 |
2022-23 |
||||
|
Pathology |
576822 |
789889 |
||||
|
Microbiology |
388820 |
373695 |
||||
|
Biochemistry |
971443 |
1376424 |
||||
|
Radiology |
74857 |
117781 |
||||
|
Neurology |
1608 |
2782 |
||||
|
Cardiology |
26654 |
43874 |
||||
|
CTVS |
138 |
145 |
||||
|
General Medicine |
13265 |
14690 |
||||
|
Urology |
1813 |
3493 |
||||
|
Orthopaedics |
1410 |
1401 |
||||
|
Gynaecology |
4582 |
7726 |
||||
|
ENT |
2125 |
5394 |
||||
|
Dermatology |
892 |
2836 |
||||
|
Blood Bank |
3585 |
4348 |
||||
|
|
2021-22 |
2022-23 |
||||
|
Diet Counseling OPD |
1975 |
3327 |
||||
|
Diet Counseling IPD |
2556 |
3979 |
||||
|
Diet Supply |
96388 |
122114 |
||||
|
|
||||||
|
Hospital Charges collected |
2021-22 |
2022-23 |
||||
|
Total Investigation Charges |
6,84,62,447.00 |
8,58,20,870.00 |
||||
|
Lamination Fees |
22,500.00 |
21,060.00 |
||||
State of Art Radiology & Imaging Department exist with various radiology equipments including 128 slice CT Scan, 1.5 Tesla MRI, Digital Radiography and Digital Radio Fluoroscopy System, various advance Ultrasonography Systems, Mobile DSA system, Mammography, DEXA Scan.
The Institute also has the following High end Medical Equipments including Cathlab, Lithotripsy Machine, Trans Esophageal Echocardiography, Stereotactic Navigational System for Neuro Surgery, CUSA, ECMO, Holmium LASER, Virtual Dissection Table, Virtual Autopsy System.
The hospital has got a Gas Manifold System with centralized pipelines. It has got the following Oxygen supply System:
- 2 Nos. Liquid Medical Oxygen Plant each having 10 KL capacity.
- 2 units of PSA (Pressure Swing Adsorption) plants each having capacity of 300L/min and 700 L/min respectively
Health Schemes:
NEIGRIHMS has been successfully operating the central schemes like RAN, HMDG, JSY, JSSK, PMNRF and also the Government sponsored Insurance Schemes like Pradhan Mantri Jan Arogya (PMJAY). The Institute entered into an agreement with Government of Arunachal for having cashless treatment for the people of Arunachal at NEIGRIHMS under the Chief Minister Arogya Arunachal Yojana (CMAAY) Scheme (Health Insurance Scheme).
NEIGRIHMS also received the award “Highest Number of Claims” among smaller States for PMJAY.
Hospital Statistics Report (APRIL 2022 to March 2023)
Grant-In-Aid And Budget
The Institute receives Grants-in-Aid from the Ministry as shows in the table below. Besides the Grants-in Aid the Institute also receives Grants from ICMR, DBT etc for implementation of various projects.
|
Budget Estimate for 2023-24 |
Allocation for 2023-24 |
Funds release by MoHFW as on November 2023 |
|
662.88crs |
528.83crs |
290.21crs |
Major Expansion Projects of NEIGRIHMS
The Hon’ble Union Minister of Health & Family Welfare and Chemical and Fertilisers inaugurated the following projects at the Institute on 14.10.2023
- Under Graduate Medical College for 100 intake with Hostels.
- Regional Cancer Centre with 252 bed capacity and Patient Guest House
- New Nursing College for 100 intake with Hostels.
- Modular OTs – 8 Nos.
- Virtual Autopsy
- Laying of foundation for 150 beds Critical Bedded Hospital Block
Capacity of Buildings etc: –
|
Facility |
Capacity |
|
Nursing College |
100 students/ year |
|
Nursing Hostel-1 |
68 Rooms, Capacity 136 students |
|
Nursing Hostel-2 |
87 Rooms, Capacity 174 students |
|
Nursing dining |
Capacity-390 seats |
|
Guest House |
28 Rooms, Capacity 52 persons |
|
UG Hostel-1 |
76 Rooms, Capacity 92 students |
|
UG Hostel-2 |
91 Rooms, Capacity 109 students |
|
UG Hostel-3 |
91 Rooms, Capacity 109 students |
|
UG Hostel-4 |
74 Rooms, Capacity 88 students |
|
Internee Hostel |
84 Rooms, Capacity 100 students |
Regional Cancer Centre with 252 bed capacity with Patient Guest House of 28 rooms will have all state of art cancer diagnosis and treatment facilities including Linear Accelerators, Brachy Therapy, CT Simulator, PET CT-Scan, Gamma Camera and various advanced cancer treatment facilities with 6 Modular Operation Theatres. It is also have all state of the are diagnostic radiological equipments including 3 Tesla MRI, 128 slice CT Scan, Digital Mammography, DSA, DRF, DR systems and PACS (Picture Archiving and Communication System). There will be Oncology, Onco Surgery, Radiation Therapy and Oncolmaging facilities.
Status of Construction of Critical Care Block (Ccb) with 150 Beds:
Ministry has approved the award of work vide F.NO. G-2017/10/2021-NE dated 10th Jan 2023, for Rs 86,52,21,005.16, to M/s SPD Construction Ltd.
Institute conveyed approval to HSCC vide NEIGR/ENGG/30/2022 dated 16th Jan 2023. Notification of Award issued to M/s SPD Construction Ltd vide No. HSCC/Neigrihms/CCB/2023 dated 17.01.2023. Time of Completion -15 calendar months from date of commencement.
Acquisition of Additional Land:
The District Collector, East Khasi Hills Revenue, has formally handed over the 20 Acres of Additional Land to NEIGRIHMS, on the 23rd November 2020, for construction of Dwelling Units for Faculty, Group A, B & C categories.
Institute has requested M/s HSCC to prepare the DPR which shall include the following:-
|
Type |
Nos |
Pay Scale |
Description |
|
VI |
20 |
10000 + Above |
3-BHK + Servants Room |
|
V |
157 |
7600 + Above |
3-BHK + Servants Room |
|
IV ( Special ) |
112 |
6600 + Above |
3-BHK + Servants Room |
|
IV |
300 |
5400 + Above |
3-BHK |
|
III |
165 |
4200 -4800 |
2-BHK |
|
II |
90 |
1900-2800 |
2-BHK |
- Boundary Wall
- Public amenities / shopping complexes
- Parks, parking
- Sports facilities (Tennis courts, basketball , football )
- Road networks, Internal and external electrification
- RCC – Overhead water tank of at least 5.00 lakhs capacity for gravity distribution to all dwelling units along with filters etc
- Effluent treatment plant ( ETP) + Sewerage treatment plant ( STP)
- Rain water harvesting, PHE works etc
- Regional Institute of Paramedical and Nursing Sciences (RIPANS), Aizawl, Mizoram
Regional Institute of Medical Sciences was set up in 1972 and has been functioning under the Ministry of Health and Family Welfare since 1 st April, 2007. RIMS is an Institute of regional importance catering to the needs of the North Eastern Region in the field of medical education by providing undergraduate and post-graduate courses. RIMS, Hospital is a 1,200 bedded teaching Hospital equipped with modern state of the art equipment and teaching facilities. The Hospital provides services to a large number of patients both out-door as well as indoor patients and admit over forty thousand patients in a year. The institute has so far produced 3848 medical graduates and 2357 specialists.
At present, the institute is conducting the following Courses:
|
Sl. No. |
Name of Course |
Duration |
|
1. |
B.Sc .Nursing |
4 years |
|
2. |
B.Sc. MLT (Medical Laboratory Technology) |
4 years |
|
3. |
B. Pharm |
4 years |
|
4. |
B.Sc.RIT (Radio Imaging Technology) |
4 years |
|
5. |
B. Optometry |
4 years |
|
6. |
M.Pharm |
2 years |
|
7. |
M.Sc.MLT |
2 years |
|
8. |
M.Sc Nursing |
2 years |
Achievements (as of 19.12.2023):
- Total strength of students in various Courses, students newly admitted and number of passed out students is shown as under:
|
Sl. No. |
Name of Course |
Total strength of students |
No. of students newly admitted |
No. of students passed out in |
|
|
|
|
in 2022-23 |
2022 |
|
1. |
B.Sc. Nursing |
171 |
53 |
39 |
|
2. |
B.Sc. MLT (Medical Laboratory Technology) |
147 |
39 |
32 |
|
3. |
B. Pharm |
155 |
40 |
30 |
|
4. |
B.Sc. RIT (Radio Imaging Technology) |
134 |
37 |
29 |
|
5. |
B.(Optometry) |
128 |
36 |
31 |
|
6. |
M. Pharm |
40 |
22 |
19 |
|
7. |
M.Sc.MLT |
16 |
8 |
|
|
8. |
M.Sc Nursing |
25 |
25 |
|
|
TOTAL |
816 |
260 |
180 |
|
- About 99% of passed out students are getting placement in various Central/State Government Institutes/Departments and private establishments such as CSIR Laboratories, AIIMS, Safdurjang Hospital, NEIGRIHMS, RIMS, NIPER, GNRC, AMRI Hospital, Apollo Hospital, Birla Heart Institute, Fortis Hospital, TATA Hospital, NIT, Mizoram University, Assam Downtown University, Assam Technical University, NATCO Pharma Ltd., Torrent Pharmaceuticals Ltd., CIPLA, etc. and abroad such as Australia, USA, Canada, Ireland, England, Norway, Singapore etc.
In addition, many students qualified for the All-India GPAT examination conducted by National Testing Agency (NTA).
- As per instruction of Ministry, recruitment process for filling up of various vacant posts at RIPANS is being initiated and action plan is being updated from time to time.
Project of Development of RIPANS:
-
- Approval for the Project of Development of RIPANS at an estimated cost of Rs. 480.12 crore was conveyed by the Ministry on 27.02.2019.
- Approval to award the work to the lowest bidder at Rs. 217.97 crore was conveyed by the Ministry on 04.01.2022
-
- Civil construction work was started on 01.03.2021 and the scheduled date of completion is 28.08.2023
-
-
- The main components of the project are as under:
Construction of: –
|
Sl No. |
Description |
Main Components |
Progress Status in percentage |
Remarks |
|
1 |
Guest House (G+3 floors) |
10 Units |
98% |
Testing and commissioning work in progress. Furniture to be installed. |
|
2 |
100 bedded Hospital Block (G+4 floors) |
100 Bedded |
93.50% |
Finishing & MEP work in progress. |
|
3 |
Staff/Nurse quarters (G+5 floors) |
18 Units |
95.10% |
Finishing & MEP work in progress. |
|
4 |
General Hostel Block (G+5 floors) |
168 Single rooms |
94.95% |
Finishing & MEP work in progress. |
|
5 |
Resident Doctor’s Quarter (G+5) |
22 Units
|
85.65%
|
RCC in superstructure, Finishing & MEP work in progress. |
|
6 |
Academic Block-IV (G+5 floors) |
18 Classroom & 32 Labs |
77.80% |
Structure, finishing & MEP work in progress. |
|
7 |
Indoor Sports Complex and Auditorium- structure work (G+3 floors) |
1000 sitting capacity |
45.20% |
RCC in superstructure in progress. |
|
8 |
Medical Superintendent Quarter (G+1) |
Single Unit |
57.10% |
RCC in superstructure in progress. |
The overall physical progress is 81.90%.
- Opening of 7 new Courses
- Creation of 154 posts.
- Financial Position during the year 2022-23:
|
(Rs. In Crore) |
||||||||
|
S. No . |
Particulars |
B.E. (In crore) |
Unspent Balance of the previous year |
Amount released by the Ministry |
Internal Resources Generated |
Expenditu re as on 31.03.2023 |
Unspent balance as on 31.03.2023 |
|
|
1 |
GIA General |
15.00 |
0.00 |
15.00 |
– |
15.00 |
– |
|
|
2 |
Grants for Creation of Capital Assets |
102.50 |
0.00 |
79.00 |
– |
79.00 |
0.00 |
|
|
3 |
GIA Salaries |
14.50 |
0.00 |
14.50 |
0.26 |
13.49 |
1.27 |
|
|
|
Total |
132.0 |
0.00 |
108.50 |
0.26 |
108.76 |
1.27 |
|
Note: Unspent Balance of the previous year was remitted to consolidated fund of India.
- Financial Position during the year 2023-2024 (upto 19..12.2023:
(Rs. in Crore)
|
Sl. No. |
Particulars |
B.E. (In crore) |
Unspent Balance of the previous year |
Amount released by the Ministry |
Internal Resources Generated |
Expenditure as on 19.12.2023 |
Unspent balance as on 19.12.2023 |
|
1 |
GIA General |
16.00 |
0.00 |
12.00 |
– |
11.22 |
0.78 |
|
2 |
Grants for Creation of Capital Assets |
93.54 |
0.00 |
71.28 |
0.00 |
55.95 |
15.33 |
|
3 |
GIA Salaries |
15.50 |
0.00 |
11.00 |
0.00 |
8.51 |
2.49 |
|
TOTAL |
125.04 |
0.00 |
94.28 |
0.00 |
75.68 |
18.60 |
|
The courses being run along with intake capacity in the institute are as follows:
2.1 Allocation of Seats for undergraduate courses:
The number of annual admissions to MBBS course is 125 students. The detail of these seats is as under:-
|
Sl. No. |
Name of State |
MBBS |
BDS |
B.Sc. Nursing |
|
1 |
All India Quota |
19 |
7 |
– |
|
2 |
Arunachal Pradesh |
7 |
4 |
5 |
|
3 |
Meghalaya |
13 |
7 |
5 |
|
4 |
Mizoram |
7 |
4 |
5 |
|
5 |
Manipur |
30 |
13 |
20* |
|
6 |
Sikkim |
5 |
3 |
5 |
|
7 |
Tripura |
13 |
7 |
5 |
|
8 |
Nagaland |
10 |
5 |
5 |
|
9. |
NE Open- All Beneficiary states of RIMS (except Assam) |
10 |
– |
– |
|
10. |
EWS |
11 |
– |
– |
|
Grand Total |
125 |
50 |
50 |
|
* including 4 seats earmarked for children of RIMS employees.
2.2 Distribution of P.G. seats 50% (73-74) seat distribution of Beneficiary States of RIMS, Imphal
|
Course |
State |
No. of seats |
Total seats |
|
|
Sponsored |
Open |
|||
|
Postgraduate (MD/MS/DCP) |
Arunachal Pradesh |
8 |
2 |
10 |
|
Manipur |
8 |
2 |
10 |
|
|
Meghalaya |
8 |
2 |
10 |
|
|
Mizoram |
8 |
2 |
10 |
|
|
Nagaland |
8 |
2 |
10 |
|
|
Sikkim |
8 |
2 |
10 |
|
|
Tripura |
8 |
2 |
10 |
|
|
RIMS AIQ Graduate |
|
2 |
2 |
|
|
NON RIMS Graduates of beneficiary States (except Assam) |
|
5 |
5 |
|
|
NE open (Graduates of beneficiary states of RIMS except Assam) |
6 |
6 |
||
|
*This Category was made available only for 2022 session due to increased of PG seats. |
||||
|
83 |
||||
2.3 Academic Achievement
The objective of this premier institute is to impart quality medical education and has produced a number of medical doctors/specialists and health care providers. On the basis of the record maintained by the institute number of the students passed out so far as on 30.09.2023 is as under:
|
1 |
Total no. of MBBS doctors passed out |
3848 |
|
2 |
Total no. of MD/MS/DCP passed out |
2357 |
|
3 |
Total no, of M.Ch./students passed out |
28 |
|
4 |
Total no. of M.Phil. (Clinical psychology) |
79 |
|
5 |
Total no. of B.Sc. (Nursing) Passed out |
376 |
|
6 |
Total no. of B.D.S. passed out |
210 |
|
7 |
B.Sc. (MLT) passed out |
5 |
3. Management of the Institute
The overall management of the institute is entrusted to i) Board of Governors headed by Union Minister of Health and Family Welfare and ii) Executive Council headed by the Secretary Ministry of Health & Family Welfare, Govt. of India.
The Board of Governors is the highest and ultimate authority of the Institute which has the power to do all necessary acts for the attainment of the objects of the Institute specified in the Memorandum of Association. The management and administration of the Institute such as supervising the overall administration and management of the Institute, review of the finance of the Institute, review of the progress of the work of the Institute, approval of the academic, scientific and technical programmes of the institute, framing Bye-laws and procedures for conduct of the affairs of the Institute etc. vest with the Executive Council. The other committees have also been constituted such as Standing Finance Committee, Academic Sub-Committee etc. for financial, academic matters.
The Director RIMS, Imphal is the Chief Executive Officer who acts as Head of all academic, scientific and administrative functions of the institute and is the overall in-charge of day to day affairs of the Institute.
The Medical Superintendent is the overall in-charge of the hospital, who looks after the day to day functioning of the hospital. The functioning of the different departments is directly under the respective heads of department. Key areas such as the Casualty, CSSD, Stores, Hospital Waste Management, etc are looked after by designated officers (medical doctors) under the supervision of the Medical Superintendent.
4. Staff Strength In RIMS
|
Sanctioned Posts |
Filled |
|
1936 |
1366 |
5. Newly Procured Equipments/Instruments
The list of newly procured major equipments for RIMS Imphal for the year 2023-2024 are as follows:
- Fundas Camera with FFA, used for the screening, diagnosis, documentation and surveillance of retinal, optic nerve and retinal vascular abnormalities and Automated Static Perimetry, an indispensable tool in the diagnosis of glaucoma, were procured and installed at Ophthalmology Department on 19/05/2023.
- Synthetic Cadaver was installed at Anatomy Department, RIMS, Imphal.
- EMG Based Simulator was installed at Physiology Department, RIMS, Imphal.
- HD Bronscopy and Stroboscopy Set was installed at ENT Department.
6. Other Achievements
- Mess Hall for Gents PG Hostel No. 2, RIMS, Imphal was inaugurated on 02/05/2023.
- 30 bedded PG Hostel for Gents, RIMS, Imphal was inaugurated on 02/05/2023
- 100 Bedded Hostels for OBC Girls, RIMS, Imphal was inaugurated on 06/04/2023.
- 100 Bedded Hostels for OBC Boys, RIMS, Imphal was inaugurated on 06/04/2023.
8. Budget
|
Budget Estimate or FY 2022-23. |
Budget Allocation for FY 2023-24 |
|
560.00 |
629.16 |
(Rs. in crore)
- Food Safety and Standards Authority of India (FSSAI):
- Food Safety and Standards (FSS) Act, 2006 was enacted with the objective to consolidate the laws relating to food and for laying down science based standards for articles of food as well as to regulate their manufacture, storage, distribution, sale and import to ensure availability of safe and wholesome food for human consumption. The Food Safety and Standards Authority of India (FSSAI) was established in September, 2008 under the provisions of the FSS Act as the apex authority on all matters of food safety and to ensure safe and wholesome food to consumers.
- FSSAI has constituted 21 subject specific Scientific Panels under Section 13 of the FSS Act, which consist of independent scientific experts, to act as the risk assessment bodies and provide their considered scientific opinion. There is also a Scientific Committee under Section 14 of the FSS Act. The Scientific Committee and the Scientific Panels provides scientific opinions and recommendations on development of food standards.
- During 2023, FSSAI continued to work towards development/revision of science based standards of food products. During 2023, FSSAI notified 4 final notifications and 3 draft notifications. The final notifications include standards/revised standards for various articles of food viz basmati rice, fowl eggs, lowering of fat content for double toned milk, limits of naturally occurring formaldehyde in freshwater and marine fish, standards for sheep milk, oils, desiccated coconut, wheat flour or resultant wheat flour, millets, mithun (Bos frontalis), dried sweet marjoram, coconut neera, liquid nitrogen dosing in ‘Natural Mineral Water’ and ‘Packaged Drinking Water’, substances added to food, microbiological standards etc.; allow the declaration of nutritional information such as calorie and carbohydrate content on alcoholic beverages and revision in the definition of single malt or single grain whisky; revision of sitting fee for members of the Food Authority other than ex-officio members. Draft notifications include revised limits of Selenium, Manganese, Iron and Biotin in Foods for Infant Nutrition; omission of AGMARK certification mark; Standards of Mead (Honey wine), Craft Beer, Indian liquors and definition of Low Alcoholic Beverages/ RTD, Wine based Beverages and, Country Liquors etc.
- FSSAI also notified one new FSS Regulation viz Food Safety and Standards Authority of India (Financial) Regulations, 2023; one amendment in FSS rules relating to Notification with respect to Qualification of Designated Officer, Duties of Food Safety Officer, Forms etc. and a Notification in exercise of the powers conferred under clause (ii) of sub-rule 1 of rule 2.1.3 of the FSS Rules with respect to Qualification for the post of Food Safety Officer.
- All Food Business Operators (FBOs) in the country are required to be registered or licensed under Section 31 of the FSS Act, 2006 to run any food business. The process for applying and issuance of licenses and registration of FBOs is completely online through Food Safety Compliance System (FoSCoS). As on 30.11.2023, 64,803 Central Licenses, 10,06,837 State licenses and 45,83,408 Registrations are active. Adding Licenses and Registration granted at Railways Stations and Airports/Seaports, the cumulative figures for the active license and registration becomes 56.63 lakhs. Continuous steps are being taken to simplify the procedure of licensing and registration of food businesses and digitation of enforcement activities.
- FSSAI, vide order dated 11-01-2023, has decided that all the renewal of license/ registration will be granted instantly, without requiring the scrutiny/approval of the concerned authority. FSSAI, vide order dated 13-01-2023 has mandated that all Manufactures (including Repacker and Relabellers) shall, through FoSCoS, upload six monthly lab testing reports or link such reports from InFoLNet wherever the samples are analysed by FSSAI notified labs. FSSAI, vide order dated 10-02-2023 has reduced the initial application fee for FSSAI license to Rs. 1000/- plus GST [as applicable]. Further, to bridge the Gender Gap and to foster equal opportunities by facilitating the participation of Women and Transgender entrepreneurs in the food business sector, FSSAI has created the provision in FoSCoS for the categorization of their applications for license/registration under the ‘Special Category’ and directed authorities to process such applications in 1:1 ratio with ‘General Category’ application.
- FSSAI is extending both technical and financial support to the States/UTs for strengthening the Food Safety Ecosystem in the Country through MoU. During 2023-24, as of 14.11.2023, funds to the extent of Rs. 360.41 crore have been approved based on work-plan proposals received from 21 States/UTs and an amount of Rs.144.83 crore released to 16 States/UTs as mainly first tranche against the work plans finalized in consultation with States/UTs.
- FSSAI has developed the State Food Safety Index to measure the performance of States on various parameters of Food Safety. The index is based on performance of States/UTs on six significant parameters, namely (i) Human Resource and Institutional Data; (ii) Compliance; (iii) Food Testing Infrastructure and Surveillance; (iv) Training and Capacity Building; (v) Consumer empowerment; and (vi) Improvement in SFSI Rank. The 5th State Food Safety Index for the year 2022-2023 was released on 7th June 2023, on the occasion of World Food Safety Day. Among larger States, Kerala secured the first rank followed by Punjab and Tamil Nadu. Among Smaller States, Goa secured the first rank followed by Manipur and Sikkim. Among the UTs, Jammu and Kashmir got the first rank followed by Delhi and Chandigarh.
- During 2023, 11 food laboratories have been recognized/notified and 03 food testing laboratories de-notified under Section 43(1) of FSS Act, 2006 by FSSAI. This has raised the total number of notified food laboratories for primary food sample testing to 239 till date. In addition, 22 Referral Laboratories have been recognized/notified under Section 43(2) of FSS Act, 2006 by FSSAI for appellate food sample testing. Out of these labs, 55 Food Testing Laboratories have obtained recognition from Agricultural and Processed Food Products Export Development Authority (APEDA) for testing of organic food products.
- FSSAI has granted approval (Order dated 25.08.2023) to the 11 food laboratories as National Reference Laboratories (NRL) in accordance with Regulation 3 of the Food Safety and Standards (Recognition and Notification of Laboratories) Regulations, 2018, for specified areas mentioned alongside each NRL and one (1) as an Ancillary National Reference Laboratory (ANRL).
- As on date, FSSAI has sanctioned 405 Mobile Food Testing Laboratories i.e Food Safety on Wheels vehicles (FSW) and out of which 238 are deployed across the nation. Total 175934 food tests, 10062 awareness and 5108 training activities have been conducted through FSWs during this period.
- The Scientific Panel on Methods of Sampling and Analysis has formulated test methods for detection of Iron, Folic acid and Vitamin B12 in Fortified Rice, Fortified Rice Kernel and Premix for FRK, which were approved by Scientific Committee and Food Authority and published vide order dated 08.09.2022, 07.11.2023 and 09.11.2023, respectively. Till date, FSSAI has notified 42 laboratories for testing of Fortified Rice, 21 laboratories for Fortified Rice Kernel and 10 laboratories for Vitamin-Mineral Premix for FRK. FSSAI has formulated guidelines on sampling of Fortified Rice (FR), Fortified Rice Kernels (FRK) and Vitamin Mineral Premix for FRK
- Total 42 physical training programmes were organized by FSSAI during 2023 in coordination with Referral Laboratories of FSSAI on safety parameter. 516 laboratory personnel attended these trainings.
- FSSAI has been conducting Pan India Surveys of various food products. The report of the Pan India Surveillance on Jaggery & Milk has been published on FSSAI website in Jan’23. FSSAI conducted Fortified Rice Kernel (FRK) surveillance drive in May, 2023, to analyse three micronutrients viz; iron, folic acid & vitamin B12 from FRK manufacturers (state & central license holders) by involving 10 FSSAI notified laboratories. In addition, FSSAI has initiated surveillance activities on Milk & Milk Products such as Khoa, Chenna, Paneer, Ghee, Butter, Dahi /Yoghurt, ice-cream, etc. through QCI and NDDB-CALF in Aug’23.
- In 2023, FSSAI has taken several initiatives with respect to Millets. The Millets Calendar prepared by FSSAI was launched by Hon’ble Union Minister of Health and Family Welfare, Dr. Mansukh Mandaviya in January 2023.
- As a part of the celebration of IYoM 2023, the FSSAI organized the Global Millets (Shree Anna) Conference on 18th and 19th of March, 2023. During the inauguration of the Global Millets (Shree Anna) Conference, Hon’ble Prime Minister Shree Narendra Modi digitally launched a book, “Shree Anna – A Holistic Overview,” based on standards on millets prepared by FSSAI.
17. Further, FSSAI hosted the ‘Eat Right Millets (Shree Anna) Quiz’ on the MyGov platform from 3rd April 2023 to 30th June 2023 as part of the celebrations for the International Year of Millets (IYOM) 2023 and organized a 4 weeks’ Shree Anna (Millets) Challenge for more than 2100 certified Eat Right Campuses from 20th April ’23 to 20th May ’23 to encourage millet-based food products, recipes, and their benefits to people at large. A total 84 Campuses participated in the challenge. Participating Campuses encouraged millets-based food in their canteens, shared recipes and organized awareness sessions on the benefits of millets during the challenge. 5 Eat Right Campuses were awarded with Certificate of Appreciation. A Millet Recipe Book for Canteens and Mess (available in six Indian languages) and a Probiotic Recipe book were unveiled by Union Health Minister Dr. Mansukh Mandaviya.
- The World Food Safety Day was celebrated at Vigyan Bhawan, Delhi on 7th June 2023. Certificate of appreciation were awarded to Kerala and Jammu & Kashmir for doing Eat Right Millets Melas and Eat Right Melas respectively in all the districts. From January to October 2023. In 2023, 151 Eat Right Melas and 100 cyclothons/walkathons/Yoga sessions have been conducted. The objective of the melas is to educate people about the importance of including millets in their diet and to showcase different ways of using millets in food.
- To promote the use of millets-based food and healthy eating practices among the armed forces and ensure availability of safe and nutritious food, a Memorandum of Understanding (MoU) was signed between the Ministry of Defence (MoD) and the Food Safety and Standards Authority of India (FSSAI) on 13th July, 2023 in the presence of Hon’ble Raksha Mantri Shri Rajnath Singh and Hon’ble Union Minister of Health and Family Welfare, Dr Mansukh Mandaviya. A book titled ‘Healthy Recipes for Defence’ to promote the consumption of Shree Anna (millets) and its health benefits was also unveiled during the occasion. A short awareness video/TVC on millets was developed and released jointly by Hon’ble Raksha Mantri and Hon’ble Union Minister of Health and Family Welfare.
- The first ever Global Food Regulators Summit 2023 was organised by FSSAI, on 20-21 July 2023. Nearly, 70 national and international speakers, including senior officials from Codex, FAO, WHO, World Food Programme and international research institutions and Universities participated. During the event, Hon’ble Union Minister of Health and Family Welfare, Dr. Mansukh Mandaviya unveiled various initiatives including Food-o-Copoeia, a collection of food category-wise monographs. The Hon’ble Union Minister also launched the common regulators platform ‘SaNGRAH’ – Safe food for Nations: Global food Regulatory Authorities Handbook. It is a database of Food Regulatory Authorities of 76 countries across the world, their mandate, food safety ecosystem, food testing facilities, contact details for food authorities.
- Eat Right Summit 2023 was organized at Vigyan Bhawan, Delhi under the aegis of Food Safety and Standards Authority of India (FSSAI) on 31st October 2023. The theme of this summit was ‘Shri Anna’ in view of the ongoing International Year of Millets 2023.
- India has been recognized by FAO for being the host country secretariat for Codex Committee on Spices and Culinary Herbs and its contribution to Codex. Codex Alimentarius Commission has considered during conference held at Rome from 27November to 02 December 2023, the proposal of India to develop global standards for group of Millets. The Commission was informed that India has already developed a group of standards for 15 types of Millets.
- A national level training institute “National Training Centre for Food Safety and Standards” has been set up at Indrapuram, Ghaziabad in 2023. Staff Training Unit has successfully organized four induction training programmes titled Nurturing Individual Potential and Unleashing Networking (NIPUN) for the newly recruited officials/officers to acquaint them with the organizational procedures and workflow. During the period, FSSAI has conducted Induction/Refresher Training of more than 300 officers which includes Food Safety Officers, Designated Officers of various States/UTs, Indian Railway, Airport Health Organization/Port Health Organization and Central Food Safety Officers of FSSAI.
- In 2023, 9112 trainings of Food Safety Supervisors have been conducted and more than 3.03 lakh Food Handlers have been trained and assessed under Food Safety Training and Certification (FoSTaC) Programme. It is matter of great prode that more than 14 lakhs Food Handlers have been trained under the FoSTaC program during the last 6 years.
- Food imports in the country are being regulated at 161 points of entry. Presently, 62 points of entry are under the direct control of FSSAI officials through offices located at 13 places i.e. Delhi, Mumbai, Kolkata, Chennai, Tuticorin, Hyderabad, Bangalore, Krishnapatnam, Vishakhapatnam, Kandla Mundra, Kochi, Mangalore and Ahmedabad. Of the rest 99 points of entry, Custom officers have been notified by FSSAI as Authorised Officers to regulate the clearance of food consignments as per the norms prescribed by FSSAI. To ensure the safety of high-risk food products like Milk, Egg, Meat, Infant Food, Nutriceuticals etc., it was decided that these products shall be only permitted through restricted 79 points of entries. FSSAI initiated registration of Foreign food manufacturing facilities intending to export Milk, Meat, Egg Powder, Infant Food and Nutraceuticals to India. A database of registered foreign food manufacturers is created through a dedicated online portal i.e. Registration of Foreign Food Manufacturers (ReFoM) for further risk analysis. So far 47 countries have registered covering 3043 foreign food manufacturers.
- International Health Regulations
DGHS, Ministry of Health and Family Welfare is designated National Focal Point for India. Functions of NFP include: capacity building for IHR(2005) in the country, review progress of IHR implementation by using WHO IHR monitoring tool and share with WHO annually, coordination and communication with WHO, NFP of other countries and local stakeholders for event verification, notification, contact tracing(TB), etc.
During the pandemic of Covid -19, the failure of the Member States and that of WHO as a directing and co-ordinating body for the global health to protect the population from COVID-19, lead to introspection at various levels. The 74th World Health Assembly in 2021 constituted a Working Group on health emergencies preparedness and response (WGPR).The report of the WGPR was considered by World Health Assembly (WHA), led to the evolvement of two Member State driven intergovernmental processes:
The Intergovernmental Negotiating Body (INB) for developing a WHO convention, agreement or other international instrument on pandemic preparedness and response (WHO CA+).The Working group on International Health Regulations (WGIHR) to recommend targeted amendments to IHR; Both the proposed instrument and the targeted amendments to IHR are to be placed before the 77th WHA in May 2024.
INSACOG:
- The Indian SARS-CoV-2 Genomics Consortium (INSACOG) is a national multi- agency consortium of Genome Sequencing Laboratories (RGSLs) laboratories established by the Government of India on December 2020.
- The network carries out whole genome sequencing of SARS-CoV-2 virus across the nation, aiding the understanding of how the virus spreads and evolves, and provides information to aid public health response.
- A summary of the cumulative data of INSACOG and other state sequencing initiatives can be found in the INSACOG data portal along with other INSACOG related information at https://inda.rcb.ac.in/insacog/statisticsinsacog
- Presently, INSACOG is keeping a close watch and monitoring the emergence and evolution SARS CoV 2 sub-lineages.
- Currently circulating variants are: JN.1, BA.2.38, BA.2.10.1, and Recombinant Variants i.e. XBB.2.3, XBB.2.3.*, XBB.1.16, XBB.1.16.* and GE.1.
Pradhan Mantri Swasthya Suraksha Yojana (PMSSY)
The Pradhan Mantri Swasthya Suraksha Yojana (PMSSY) envisages creation of tertiary healthcare capacity in medical education, research and clinical care, in the underserved areas of the country. It aims at correcting regional imbalances in the availability of affordable/reliable tertiary healthcare services and also augmenting facilities for quality medical education in the country. The scheme has two broad components:
Setting up of All India Institutes of Medical Sciences (AIIMS); Up-gradation of existing Government Medical Colleges/Institutions (GMCIs).So far, establishment of 22 new AIIMS and 75 up-gradation Projects of existing Government Medical Colleges/Institutions (GMCIs) have been approved under this scheme.
Six AIIMS under Phase-I:
In Phase-I, a total of six AIIMS (Bhopal, Bhubaneswar, Jodhpur, Patna, Raipur and Rishikesh) were set up at an approved cost of Rs. 820.00 crore per AIIMS. These are fully operational.
All key hospital facilities and services such as Emergency, Trauma, Blood bank, ICU, Diagnostic and Pathology are functioning. The total bed capacity of the six AIIMS is at present 6329. Hospital services in these 6 AIIMS are operating in various Specialities and Super- Specialties Departments. There are 1706 PG Seats (Medical) and 750 MBBS seats in these six AIIMS.
New AIIMS under Phase-II, IV, V, VI & VII:
16 AIIMS have been sanctioned/approved by the Cabinet in subsequent phases. Following facilities and services have been made functional in these institutes:BBS classes and OPD services started at following 11 AIIMS at Gorakhpur (UP),Raebareli (UP), Nagpur (Maharashtra), Kalyani (West Bengal), Mangalagiri (Andhra Pradesh), Bibinagar (Telangana), Bathinda (Punjab) Deoghar (Jharkhand), Bilaspur (Himachal Pradesh), Guwahati (Assam) and Rajkot (Gujarat). Of these, IPD facilities on a limited scale have been started in 10 AIIMS at Raebareli, Mangalagiri, Nagpur, Kalyani, Gorakhpur, Bathinda, Bilaspur, Deoghar, Guwahati and Bibinagar. in the current financial year 3760 hospital bedsare functional in these 10 AIIMS.
MBBS classes have started in AIIMS at Vijaypur (Jammu), and Madurai (Tamil Nadu).
There are 493PG seats in 11 AIIMS ( Nagpur, Bibinagar, Bathinda, Deoghar, Mangalagiri, Raebareli, Kalyani, Bilaspur, Gorakhpur, Guwahati and Rajkot) and intake of 1287 MBBS students in 13 AIIMS (Mangalagiri, Nagpur, Kalyani, Gorakhpur, Bathinda, Raebareli, Deoghar, Bibinagar, Guwahati, Bilaspur, Vijaypur, Rajkot and Madurai) for Academic Year 2022-23.
Up-gradation of existing Government Medical Colleges /Institutes:
The Up-gradation programme broadly envisages improving tertiary health infrastructure through construction of Super Speciality Blocks / Trauma Care Centres etc. and/or procurement of medical equipment for existing Government Medical Colleges / Institution.
Since inception of the Scheme, 64 upgradation projects of existing Government Medical Colleges / Institutions have been completed, adding about 13982 Super- specialty beds including 2397 ICU beds. The Super Specialty Blocks /Trauma Centres constructed in these upgradation projects are also being used as COVID Hospital Blocks. The civil constructions of the following 6 projects have been completed during 2022-23 (upto November, 2023):
|
S. No. |
Name of the GMC/ Institute |
Name of the State |
Phase |
Type of facility |
Total Beds |
ICU Beds |
No. of Super Specialties |
|
1 |
Indira Gandhi Medical College, Shimla |
Himachal Pradesh |
III |
SSB |
283 |
53 |
10 |
|
2 |
TD Medical College, Alappuzha |
Kerala |
III |
SSB |
279 |
62 |
9 |
|
3 |
North Bengal Medical College, Siliguri, Darjeeling |
West Bengal |
III |
SSB |
255 |
89 |
8 |
|
4 |
GMC, Kanpur |
Uttar Pradesh |
IV |
SSB |
270 |
30 |
12 |
|
5 |
GMC, Jaipur |
Rajasthan |
IV |
SSB |
231 |
51 |
5 |
|
6 |
GMC, Bhavnagar |
Gujarat |
IV |
SSB |
193 |
47 |
6 |
Setting up of Critical Care Hospital Blocks:
In order to avoid mixing of COVID and non COVID patients, at many places full hospitals were required to be designated as COVID dedicated facilities. To control spread of the infectious disease, The PM-ABHIM Scheme envisages establishment of 150 bedded Critical Care Hospital Blocks (CCHB) in 12 Central Hospitals under the Central Sector Component at a total cost of Rs. 2220 crores. These institutes include – AIIMS at Delhi, Bhopal, Bhubaneswar, Jodhpur, Patna, Raipur and Rishikesh, PGIMER at Chandigarh, JIPMER at Puducherry, RIMS at Imphal, NEIGRIHMS at Shillong and IMS of BHU at Varanasi.The allocation under the scheme broadly translates to Rs. 120 crores as capital cost which includes civil construction, ICUs, HDUs, Dialysis, OTs, MGPS, CSSD, Hospital Beds and furniture and medical equipment. In addition, estimated operating expenses for salary of doctors and paramedics are Rs. 28.0 crore per annum and Rs.12.0 crore per annum for other operating expenses on running the hospital services. The recurring cost will however be available after CCHB becomes functional and only upto 2025-26, after which Institutes will be expected to meet this requirement out of their regular budget.So far, Rs. 433.69 Crore has been released to these 12 Institutes.
- National Urban Health Mission (NUHM)
National Urban Health Mission (NUHM) was approved on 1st May, 2013 as a sub-mission under an overarching National Health Mission (NHM), NRHM being the other sub-mission. NUHM envisages strengthening the primary health care delivery systems in urban areas and for providing equitable and quality primary health care services to the urban population with special focus on slum dwellers and vulnerable population. It also seeks to decongest secondary and tertiary health care facilities (District Hospitals/Sub-District Hospitals/Community Health Centre) by providing robust comprehensive Primary health care services in urban areas.
NUHM covers all cities and towns with more than 50,000 population and district headquarters and State headquarters with more than 30,000 population. The remaining cities/ towns are covered under National Rural Health Mission (NRHM). Under NUHM, UPHCs are to be established as per norm of one U-PHC for approximately 30,000 to 50,000 urban population. Also, urban Ayushman Arogya Mandir below U-PHCs on the population of 15,000-20,000 have been approved under 15th FC and PM-ABHIM. These urban Ayushman Arogya Mandir are linked to the nearest UPHC – Ayushman Arogya Mandir for administrative, financial, reporting, and supervisory purpose.
Implementation of NUHM is through the State Health Department or the Urban Local Bodies (ULBs). In seven metropolitan cities, viz., Mumbai, New Delhi, Chennai, Kolkata, Hyderabad, Bengaluru and Ahmedabad the implementation is through the ULBs. For the other cities, the State Health Department decides whether the NUHM is to be implemented through them or the other urban local bodies. So far, 1215 cities have been covered under NUHM in 35 States/UTs.
Physical Progress:
The programme is being implemented in the States/UTs for more than 10 years. It has helped in augmentation of infrastructure and human resources dedicated towards urban areas. The progress reported by the States/UT (updated up to June,2023) in the activities approved under NUHM is as follows-
(i) Progress under infrastructure
- 1,215 cities/towns are covered under NUHM
- 5,271 UPHCs & 227 UCHCS are functional,
- 4,926 UPHCs strengthened as Ayushman Arogya Mandir as per Ayushman Arogya Mandir portal as on 18.12.2023)
- 4285 urban Ayushman Arogya Mandir below UPHC are operationalized as per Ayushman Arogya Mandir portal (as on 18.12.2023)
(ii) Progress in HR under NUHM
- 4,693 Medical Officers in-position
- 341 Specialists in-position
- 8,288 Staff Nurse in-position
- 19,492 ANMS in position
- 3,228 Pharmacist in-position
- 3,548 Lab Technician in-position
- 535 Public Heath Managers in-position
- 1,623 Programme Management staff in position at State/District/City level
(iii) MHUs under NUHM
- 56 Mobile Medical Units are functional
(iv) Progress under Community Process
- 82,296 ASHAs are in position.
- 84,842 Mahila Arogaya Samiti (MAS) are formed.
As part of Ayushman Bharat, the existing U-PHCs are being strengthened as urban Ayushman Arogya Mandir to provide preventive, promotive and curative services in cities closer to the communities. So far, 4,926 U-PHCs have been converted into urban Ayushman Arogya Mandir in the States/UTs (except Delhi).
As per Ayushman Arogya Mandir portal data as on 18.12.2023, 5.04 Cr. screenings done for Hypertension, 4.16 Cr. screenings done for Diabetes and around 1.91 Cr. screenings done for Oral cancer at these Ayushman Arogya Mandirs. Similarly, these functional Ayushman Arogya Mandirs have conducted 61 lakh screening for cervical cancer and 94 lakh for breast cancer in women.
A large proportion of urban population is usually settled in congested urban settings. Accordingly, Universal CPHC is planned to be provided through Urban Ayushman Arogya Mandir and Polyclinics, by providing support for setting up of 11,024 Urban Ayushman Arogya Mandir in close collaboration with Urban Local Bodies under PM-ABHIM from FY 2021-22 to FY 2025-26. Such Urban HWCs would enable decentralized delivery of primary health care services closer to people, thereby increasing reach of the public health systems to the vulnerable and marginalized.
National Quality Assurance Standards (NQAS) were developed for urban health facilities in Year 2016 and institutional framework has been set up in all State/UTs. As of 30thNovember 2023, 423 UPHCs are NQAS certified at national level and additionally 266 UPHCs are NQAS certified at state level .
Kayakalp and Swachh Swasth Sarvatra (SSS) have been expanded to cover urban areas also and U-PHCs have been awarded Kayakalp awards. In FY 2022-23, 1771 UPHCs and 41 UCHCs achieved 70 % and above overall score in Kayakalp external assessment and qualified for incentives under the program.
Financial Progress:
Since the launch of NUHM in FY 2013-14 till the FY 2021-22, funds to the tune of Rs 8,788.48 cr and 7,165.87 cr have been allocated and released respectively to the States/ UTs for implementation of the programme activities from FY 2022-23 financial related matters have been merged with NHM.
- ICMR: Department of Health Research (DHR)
The major activities and achievements of Department of Health Research are as follows:
- Intramural Research
Intramural research is carried out through a countrywide network of 27 institutes/centres with multiple field stations, 14 ICMR Institutes work in the area of communicable diseases; 6 in Non-Communicable Diseases, 1 in diseases related to Reproductive and Child Health (RCH);1 in nutrition and nutritional deficiencies, 3 in disease related to Basic Medical Sciences including haemoglobinopathies and traditional medicine, 1 in the area of animal breeding and research and 1 is patient care and research centre. ICMR calls proposals under Intramural Research Programme. It intends to encourage high risk, high reward research from scientists. Each institute may be awarded between 3 to 30 crores for the projects on the priority areas based on need and quality of research proposals based on priority research areas
- Extramural Research
Indian Council of Medical Research (ICMR) provides financial assistance for Indian scientists working outside ICMR institutes to conduct research in the fields of medicine, public health, and allied disciplines aimed at improving health of Indians under its Description of Extramural Research Programme.
Following types of Extramural Research Grants are there:-
- Investigator Initiated Research Proposals- Small Grant: Funding upto 2 crores per project for the entire duration. The project duration will be up to a maximum period of four years.
- Investigator Initiated Research Proposals-Intermediate Grant: Funding is between 2-8 crores for the entire duration. The duration of the project will be for 4 years.
- Centers for Advanced Research (CAR): Conducting decisive research that helps in solving an important healthcare problem by an experienced research team. It can have single or multiple linked research projects with clear deliverables. Current budgetary ceiling is 15 crores per CAR. Duration of project will be for five years.
- Mission Mode Research in area of National Priority
To establish National Health Research Program in the country. Ten priority areas have been identified for solution-based research – (1) One Health, (2) AMR, (3) TB, (4) Vector Borne Diseases (Communicable Diseases), (5) Cancer, (6) Ambulatory care for NCDs, (7) Acute emergency care (NCDs), (8) Anemia, (9) Stunting and wasting, (10) Neonatal mortality (RCH & Nutrition).
- ICMR Collaborating Centre of Excellence (ICMR-CCoE) :
ICMR recognized the research groups that have conducted excellent research in the recent past as ICMR Collaborating Centre of Excellence. In order to foster collaboration between ICMR & its institutes and Centres of Excellence as well as amongst the Centres of Excellence. This shall strengthen resources of the country, in terms of information, services, research and training in support of national health development. Duration will be of 5 years.25 centres selected as ICMR Collaborating Centre of Excellence against the call titled “Request for Expression of Interest for ICMR Collaborating Centre of Excellence (ICMR-CCoE) ( July 31, 2023).
- Achievements during the year:
The major activities and achievements of ICMR are given below:
- Outbreak Response:
DHR, with its Viral Research & Diagnostic Laboratories, including the BSL-4 facility in Pune, played a pivotal role in timely detection and containment of outbreaks like COVID-19, Nipah, Zika, and H1N1.During recent Nipah Outbreak in Kerala, ICMR confirmed a Nipah virus outbreak in Kuttiyadi, Kozhikode, Kerala, on September 12, 2023. Three out of five suspected cases were positive. ICMR deployed immediate response teams for onsite diagnosis and a bat survey. A Mobile BSL-3 facility was set up within 24 hours, testing six confirmed cases with a 33.33% fatality rate. Biosafety measures were strictly followed, demonstrating a swift and effective response to manage the Nipah outbreak in Kerala.
During the COVID-19 pandemic, ICMR has tirelessly worked to provide multi-pronged solutions ranging from ramping up the testing infrastructure, monitoring the disease spread through sero-surveys and providing novel diagnostic methods to developing vaccines for ending this contagion.
- National One Health Mission (NOHM): Under NOHM, the National Institute of One Health (NIOH) is established in Nagpur, supported by a national network of BSL-3 & BSL-4 labs. The SFC for NOHM activities has been approved.
- Disease Surveillance & Monitoring:
-
-
- ICMR has initiated integrated surveillance for SARS-CoV-2 and Influenza at 30 Virus Research & Diagnostic Laboratories (VRDLs), having a total of 90 sub sites across the country. These sites regularly monitor the influenza strains (both A & B) in different parts of the country and depict the prevailing trends of Influenza A & B and their subtypes along with SARS-CoV-2. This continuous monitoring will yield important information related to new or evolving strains of Influenza, which has the greatest potential to cause outbreaks.
- National risk map with hotspots for zoonotic disease of human importance and appropriate preventive measure are under design.
- Surveillance for Hemorrhagic fevers like Kyasanur Forest Disease (KFD) and Crimean Congo Hemorrhagic Fever (CCHF) has been set up at 10 VRDLs along the states along the western ghats for KFD (Karnataka, Kerala, Goa and Maharashtra) and in the states of Gujarat, Rajasthan and Uttar Pradesh for CCHF.
- A total of 132 VRDLs have been trained and provided reagents to detect Zika virus disease in samples negative for Dengue and Chikungunya.
-
-
- Combatting Communicable Diseases:
The Communicable Diseases are being tackled in mission mode. Initiatives like MERA India (Malaria Elimination Research Alliance), India TB Research Consortium (ITRC) are bringing multiple stakeholders (national and international) under one umbrella to achieve the aim of disease elimination. DHR-ICMR has demonstrated successful implementation models for disease elimination. Comprehensive Case Management Programme (CCMP) model demonstrated that universal access to malaria diagnosis and treatment, follow-up of patients with enhanced surveillance can dramatically reduce the number of malaria cases (85%). CCMP learning experience and several best practices from CCMP have been incorporated into the existing NVBDCP programme. Vaishali Model reduced the incidence of Kala Azar to <1 case per 10,000 and is being replicated by in Saran/Siwan district of Bihar on request of state government.
DHR-ICMR has been committed towards TB elimination. To assess the true burden of TB, the National TB Prevalence survey was conducted in all states/UTs. It is committed towards providing a cost-effective and PHC friendly diagnostics, a universal TB treatment and a vaccine to achieve the target of ‘End TB 2025’. ICMR implemented the first state wise differentiated TB care model in India aimed at reducing TB deaths. The following industry technologies validated by ICMR have been recommended to National Programmes thereby promoting Make-in-India in the Pharmaceutical sector.
-
-
-
- PathoDetect TM MTB RIF & INH drug resistance kits
- PathoDetect TM MTB & NTM Detection Kits
- C-TB with QuantiFERONR-TB Gold Plus and 2.T.U Tuberculin PPD RT23 SSI for detection of TB infection in general and key population AI TB: ICMR has uploaded 2128 Tb and 1147 non-TB annotated X-rays to train the software to diagnose TB through Artificial Intelligence. There were 1801 TB, 389 Non-TB, and 117 normal cases from our site in Phase-I to train the AI tool. The AI tool was validated in phase II mode, and 786 cases have been uploaded.
-
-
- Sickle Cell Anemia Elimination:
Realizing the PM’s vision of Sickle Cell Anemia Elimination , ICMR has established NIIH-Centre for Research, Management and Control of Haemolglobinopathies at Chandrapur, Maharashtra to address the problem of sickle cell anemia, a major health issue in this region. The Centre screens the population for sickle cell disease, and counsel and trains various state healthcare providers for effective control of hemoglobinopathies.
- Non-Communicable Diseases (NCDs):
DHR-ICMR is addressing the rising burden of Non-Communicable Diseases at top priority level through programs like India Hypertension Control Initiative (IHCI), Mission Delhi, India Cancer Research Consortium, Stroke network etc. IHCI is ICMR’s collaborative initiative with MoHFW, WHO & state governments aimed to reduce deaths from heart attacks & strokes. It is playing a critical role in regularizing hypertension treatment across primary care facilities in 100 districts in 23 states by providing free drugs & quality treatment to 34lakh patients. The tech driven innovations in the IHCI program allowed India to track over 2 million patients digitally, with minimal burden on human resources and on clinics. 50% patients tracked in real time achieved BP control. The initiative was awarded 2022 UN Inter-Agency Task Force and the WHO special program on Primary Health Care Award during United Nation General Assembly on 21st September 2022.
- Revolutionizing Healthcare System through use of drones:
The i-Drone project has transformed the Indian healthcare system by using drones to transport vaccines and medical supplies in the challenging terrains of the North Eastern States and Himachal Pradesh. The medical supplies delivered under the i-Drone project included COVID-19 vaccines, vaccines used in routine immunization programs, antenatal care medicines, multi-vitamins, syringes and gloves. Apart from these, clinical samples for testing were also delivered from PHCs & sub centres to Regional Hospital. The drone delivery system focused on an end-to-end ecosystem for drone-based logistic transportation within the states and was the first successful example of delivering vaccines through drones from land to islands in South Asia. The longest drone flight under this project carried 3525 units of medical supplies from Mokokchung to the district Tuensang in Nagaland (approx. 40 km). A total of 18,275 units of medical supplies were delivered. The drones covered a total of 735 km in terms of aerial distance in approximately 12 hours, whereas, the total road distance for all the sites would have been around 2000 km, which might have taken 50 hours and demanded several other logistic challenges. In Himachal Pradesh, deliveries were delivered at 14,000 ft and -10°C. Drones repeatedly and accurately delivered medical supplies faster than other methods deployed for delivery and without additional risks to personnel or manned airframe.
Leveraging upon the recent liberal regulation policies in India for low-altitude airspace for drones, the current feasibility study paved the path for using unmanned drones for the delivery of life-saving medical supplies in austere environments in the future.
- Medical Device and Diagnostics Mission Secretariat:
Indian Council of Medical research (ICMR), has established a ‘Medical Device and Diagnostics Mission Secretariat’ (MDMS) at its headquarters to support and catalyze research, development and indigenous manufacturing of cost-effective medical devices and diagnostics in synergy with National Health Mission (NHM), Ayushman Bharat Health and Wellness centers.
ICMR is following a multipronged approach for facilitating technology adoption for enabling accessibility, acceptability and availability of technologies in the medical device and diagnostic sector including drafting of effective policies for promoting innovation and entrepreneurship development by Medical doctors and Institutes. It has also set-up various Centers of Excellence (CoEs) at IITs in collaboration with medical institutes for fostering technology development, with focused approach of supporting technologies aligned with national health priorities. ICMR is also facilitating preclinical, clinical evaluation and Health Technology Assessment (HTA) of such frugal indigenous technologies for facilitating technology adoption for ensuring societal impact.
Multiple indigenous technologies have been developed:
- Launched a kit for detection of hemophilia A and von willebrand disease (VWD): Cost effective, Point of care.
- A rapid detection kit for Orientia tsutsugamushi diagnosis has been developed
- KFDV Point of Care Assay in collaboration with Mol Bio Diagnostics Pvt Ltd, Goa, for detection of KFD viral RNA from clinical samples was developed.
- Developed and standardized a onetube combo RT-qPCR kit for simultaneous detection of SARS-CoV-2 and influenza viruses and distributed to VRDL network
- Sudden Deaths:
The Indian Council of Medical Research (ICMR) conducted a multicentric case-control study on sudden deaths among young adults in India, prompted by concerns about a potential link between COVID-19 infection or vaccination and such incidents. The study, spanning October 2021 to March 2023, involved 47 tertiary care hospitals and found that COVID-19 vaccination did not increase the risk of sudden death in healthy young adults. In fact, vaccination was identified as a mitigating factor, reducing sudden deaths in the 18-45 age group. The study identified other risk factors, including a family history of sudden death, prior hospitalization due to COVID-19, binge drinking, and intense, unaccustomed physical activity shortly before the untimely demise. The findings were published in the Indian Journal of Medical Research.
- MoA with AcSIR to promote Medical Research
The Indian Council of Medical Research (ICMR) and the Academy of Scientific and Innovative Research (AcSIR) have inked a transformative Memorandum of Agreement (MoA). This collaboration aims to bolster the nation’s biomedical research efforts, foster innovation, and elevate the calibre of research professionals. All the current Institutes under ICMR, ICMR HQs. and Department of Health Research (DHR), will be recognized as Associate Academic Centres of AcSIR providing avenues for Ph.D. and postgraduate students. This presents an exciting opportunity to train a substantial number of Ph.D. and postgraduate students, especially creation of physician scientists, by creating opportunities for doing PhD to those with MD or MBBS degrees.
- Pradhan Mantri Ayushman Bharat Health Infrastructure Mission (PM-ABHIM):
Pradhan Mantri Atmanirbhar Swasth Bharat Yojana scheme (now renamed as Pradhan Mantri Ayushman Bharat Health Infrastructure Mission, PM-ABHIM) with an outlay of about Rs. 64,180 Cr was launched by Hon’ble Prime Minister on 25th October, 2021, to be implemented during the scheme period from FY 2021-22 to FY 2025-26. This is the largest pan-India scheme for strengthening healthcare infrastructure across the country. The measures under the scheme focus on developing capacities of health systems and institutions across the continuum of care at all levels viz. primary, secondary and tertiary and on preparing health systems in responding effectively to the current and future pandemics/disasters.
The Pradhan Mantri Ayushman Bharat Health Infrastructure Mission targets to build an IT enabled disease surveillance system by developing a network of surveillance laboratories at block, district, regional and national levels, in Metropolitan areas & strengthening health units at the Points of Entry, for effectively detecting, investigating, preventing, and combating Public Health Emergencies and Disease Outbreaks.
Increased investments are also targeted to support research on COVID-19 and other infectious diseases, including biomedical research to generate evidence to inform short-term and medium-term response to COVID-19 like pandemics and to develop core capacity to deliver the One Health Approach to prevent, detect, and respond to infectious disease outbreaks in animals and humans.
The main interventions under the ‘Pradhan Mantri Ayushman Bharat Health Infrastructure Mission’ scheme to be achieved by FY 2025-26 are:
Centrally Sponsored Components:
- Infrastructure Support for 17,788 rural Health and Wellness Centres in 10 High Z Grants and NHM.
- Establishing 11,024 urban Health and Wellness Centres in all the States.
- 3382 Block Public Health Units in11 High Focus states. Support for other States/UTs under XV Finance Commission Health Sector Grants and NHM.
- Setting up of Integrated Public Health Labs in all districts.
- Establishing Critical Care Hospital Blocks in all districts with population more than 5 lakhs.
Progress so far under the CSS components:
- Administrative approvals have been accorded to States/UTs for an amount of Rs. 14,138.98 Crore for construction/strengthening of 7,808 Sub-Health Centres- Health & wellness Centres, 1,528 U-HWCs, 890 BPHUs, 352 IPHLs at District level and 278 CCB for FY 2021-22, 2022-23 and 2023-24.
Central Sector Components:
- 12 Central Institutions as training and mentoring sites with 150 bedded Critical Care Hospital Blocks.
- Strengthening of the National Centre for Disease Control (NCDC), 5 New Regional NCDCs and 20 metropolitan health surveillance units;
- Expansion of the Integrated Health Information Portal to all States/UTs to connect all public health labs;
- Operationalization of 17 new Public Health Units and strengthening of 33 existing Public Health Units at Points of Entry, that is at 32 Airports, 11 Seaports and 7 land crossings;
- Setting up of 15 Health Emergency Operation Centres and 2 container based mobile hospitals; and
- Setting up of a national institution for One Health, 4 New National Institutes for Virology, a Regional Research Platform for WHO South East Asia Region and 9 Biosafety Level III laboratories.
Progress so far:
- In the first year of support (2022-23), a total of 33 labs will be strengthened as per target. Fund release for 20 labs has been processed.
- Land allocation for new NIVs and One health Institute at Dibrugarh, Jabalpur, Jammu, Bangalore and Nagpur (One Health) has been completed. Contracts awarded to CPWD.
Tenders in process.
- Building comprehensive surveillance system with more than 4000 labs.
- Digitization of analytics, forecasting & early warning systems through the Integrated Health Information Platform (IHIP). It is designed to capture real-time, case-based epidemiological data of more than 33 plus health conditions.
- 37,000 new critical care beds with ICU & Oxygen.
- Health units at 50 International Points of Entry: 32 Airports, 11 Seaports & 7 land crossings.
23. National Center for Vector Borne Diseases Control
1.Malaria
-
-
- India has set the goal of achieving malaria elimination by 2030.
- India has made substantial progress in reducing malaria burden. The country has achieved a reduction of 85% in malaria morbidity and 78.38% in malaria mortality between 2015 and 2022.
- In 2022, a total of 1,76,522 malaria cases with 1,01,068 Plasmodium falciparum cases and 83 deaths were reported across the country.
- In 2023, (up to November, 2023- provisional) total 1,77,333 malaria cases with 1,14,746 Plasmodium falciparum cases and 32 deaths were reported across the country.
- In 2022, National Annual Parasite Incidence (API) (Number of malaria cases per 1000 population), was 0.13 per 1000 population and total 34 States/UTs have achieved API < 1, except 2 States Tripura (3.25) and Mizoram (8.90).
- In 2022, a total 128 districts in the country have reported ‘zero malaria cases.
- In 2022, out of 758 districts only 27 districts (Chhattisgarh (3), A&N
- Island (1), Jharkhand (2), Maharashtra (1), Meghalaya (1), Mizoram (4),Odisha (5), Tripura (5) and West Bengal (1) have district API > 1 per 1000 population.
- Implementation of Integrated Health Information Platform (IHIP) for real time monitoring of malaria surveillance. GoI has onboarded all the 36 States/UTs completely in IHIP for Malaria module.
- In 2022, total 11,45,408 of LLIN were supplied to Chhattisgarh and in 2023, total 1,14,541of LLINs were distributed to Tripura.
- In FY 2023-24, 112 Lakh LLINs are under distribution for Madhya Pradesh, Chhattisgarh and Tripura and 46 Lakhs LLINs distribution is in pipeline under GFATM for 7 NE states and 1.63 crore LLINs are to be procured under DBS of 24 States.
-
- NCVBDC displayed journey towards malaria elimination, achievements of the Malaria program during the Asia Pacific Leaders’ Conclave on Malaria Elimination 2023 held in New Delhi on April 24, 2023, to sensitise the national and global audience.

1.2 Kala-Azar
- During 2023 up to November end 562 Kala-azar cases have been reported in comparison to 851 cases reported during corresponding period of 2022, reporting a reduction of 34% of cases.
- Out of 633 endemic blocks only one block was reported > 1 case per 10,000 population at block level during 2022 and as of 2023 November, none of the endemic block reported incidence more than 1 case per 10,000 population at block level.
- Mann Ki Baat on Elimination of Kala-azar by Hon’ble Prime Minister of India on 25th December 2022 and dissemination of Mann Ki Baat video during Ratri Choupal initiative in high endemic villages. Hon’ble HFM reviewed Kala-azar with Health Ministers of 4 Kala-azar endemic states on 4th January 023.NCVBDC showcased the progress of Visceral Leishmaniasis Elimination in India during the exhibition at G20 First Health Working Group Meeting, Trivandrum, Kerala on 18th & 19th January 2023 and at G20 Health Ministers Meeting held in Gujarat on 9th and 11th August 2023.

24. National Ambulance Services (NAS):
- As on date, 34 States/UTs have the facility where people can dial 108 or 102 telephone number for calling an ambulance. Dial 108 is predominantly an emergency response system, primarily designed to attend to patients of critical care, trauma and accident victims etc. Dial 102 services essentially consist of basic patient transport aimed to cater the needs of pregnant women and children though other categories are also taking benefit and are not excluded. Janani Shi6hu Suraksha Karyakram (JSSKJ) entitlements e.g. free transport from home to facility, inter facility transfer in case of referral and drop back for mother and children are the key focus of 102 service.
This service can be accessed through a toll-free call to a dedicated call centre. As on 3Oth June. 2023, 2957 also 14,613 BLS, 4259 PTV, 17 Boat and 81 Bike, Emergency Response Service Vehicles are supported under NHM, besides 6936 empanelled vehicles for transportation of patients, particularly pregnant women and sick infants from home to public health facilities and back.
25.National Mobile Medical Units (NMMUS):
Support to Mobile Medical Units (MMUs) under NHM, now encompassing both NRHIVI and NUHM, is a key strategy to facilitate access to public health care particularly to people living in remote, difficult under-served and unreached areas. As on 30th June, 2023, States/UTs have 1,525 mobile medical units which includes mobile medical units, mobile health units, mobile medical/health vans, boat clinics, eye vans/ mobile ophthalmic units, dental vans under NRHM and NUHM.
26 .24 X 7 Services and First Referral facilities
To ensure service provision for maternal and child health, 24×7 services at the PHCs have been made available. As on 30th June 2023, 12369 PHCs have been made 24×7 PHCs and 3095 facilities (including 698 DH, 857 SDH and 1377 CHCs & 163 other level) have been operationalized as First Referral Units (FRUs). Besides, NHM envisages provision of assured and high-quality maternal and child health services to be delivered with dignity and care at public health institutions. Gol launched MCH wings to facilitate assured admission for institutional delivery for all pregnant women. These wings are equipped with obstetric HDUs, lCUs, maternity OT, Labor rooms ensuring respectful maternity care etc. for managing high-risk pregnancies and those requiring C-sections. These centres also have skill labs for training of nurses and doctors for providing high quality and skilled maternity care.
*******
MV