1. Steps taken by the Government of India for COVID-19 containment and management
The Government of India continued to closely monitor the evolving nature of COVID-19 pandemic in India as well as globally. A close watch was also kept on improving knowledge about the virus, the disease, its long-term impacts, advancements being made in India as well as globally in terms of public health tools, diagnostics, therapeutics and vaccines. The various technical bodies under various Ministries/Departments continued to maintain a close watch over the evolving nature of the causative virus and their public health implications. India continued its graded yet pre-emptive and proactive approach towards COVID-19 management.
The COVID-19 trajectory in India experiences a sharp increase during March-May 2021, however, since May 2021, the trajectory has witnessed a considerable and sustained decline. As on 17th December 2021, Five States (Kerala, Maharashtra, Tamil Nadu, West Bengal and Karnataka) are contributing to close to 80% of all active cases in the country. Owing to Government of India’s five-fold strategy of test-track-treat-vaccinate and COVID appropriate behavior through a Whole of Government & Whole of Society approach, India has been able to limit its cases and deaths per million to 25,158 cases per million and 345 deaths per million population (as on 17th December 2021) respectively, which is one of the lowest in the world as compared to similarly affected countries.
The Hon’ble Prime Minister provided the much required strong and decisive leadership and guidance for national response to the pandemic. The Prime Minister Office and Ministry of Health & Family Welfare has been in regular interactions with the all States and UT administrations to review the preparedness and response measures being taken and also to identify areas for further improvement and coordination. The Committee of Secretaries under Cabinet Secretary took regular reviews with all related Ministries of Health, Defence, Ministry of External Affairs, Civil Aviation, Home, Textiles, Pharma, Commerce and other officials including with State Chief Secretaries.
Ministry of Home Affairs, Govt. of India, under extant provisions of Disaster Management Act, 2005 had constituted 11 Empowered Groups on 29th March 2020 for fast-tracking informed decision making for COVID-19 management. Based on the evolving needs and scenario in the country, on 11th September 2020, these groups were condensed into six larger empowered groups (EGs). On 29th May 2021, these were reconstituted in 10 Empowered Groups. These 10 Empowered Groups are tasked with (i) Emergency Management Plan and Strategy, (ii) Emergency Response Capabilities, (iii) Augmenting Human Resources and Capacity Building, (iv) Oxygen, (v) Vaccination, (vi) Testing, (vii) Partnership, (viii) Information, Communication and Public Engagement, (ix) Economic and Welfare Measures and (x) Pandemic Response and Coordination.
The Health Ministry continues to work closely with the States and has held regular video conferences with States regularly. 118 video conferences were held with State Health Ministers, State Health functionaries and district level officials. The Joint Monitoring Group (JMG) under the Chairmanship of DGHS and National Task Force on COVID-19 under ICMR continue to assess the risk, review the preparedness & response mechanisms and finalize technical guidelines.
The Government of India, based on its past experience of successfully managing pandemics and epidemics in the past and the evolving evidence based contemporary knowledge about the disease, provided the requisite strategy, plans and procedures to the State Governments and UT administrations. This includes containment plans and guidelines on a wide range of subjects related to travel, behavioral & psycho-social health, surveillance, laboratory support, hospital infrastructure, clinical management, rational use of Personal Protective Equipment (PPE) etc.
Taking note of the evolving COVID-19 situation globally and emergence of mutant variants of SARS-CoV-2 virus, the guidelines for international arrivals were reviewed from time to time. The last updated guidelines were issued on 30th November 2021. As per the guidelines, regions/countries have been re-classified as ‘at-risk’ based on the epidemiological situation of COVID-19 in these regions/countries and/or reporting of Omicron variant from these countries. List of such ‘at-risk’ regions/countries is dynamic in nature and has been updated from time to time.
All travelers coming from countries deemed ‘at-risk’ will also mandatorily undergo COVID-19 testing on arrival through RT-PCR, followed by mandatory home quarantine for 7 days. A repeat RT-PCR testing shall also be done on 8th day of arrival in India to be monitored by State Health Authorities. Two percent of travelers from ‘non-at-risk’ countries will be tested at random for COVID-19. Individuals tested positive shall be subjected to Whole Genomic Sequencing at identified INSACOG network laboratories to determine the presence of SARS-CoV-2 variants (including Omicron).
Union Ministry of Health & FW is coordinating and collaborating with other stakeholder Ministries/departments including Ministry of Civil Aviation, Ministry of Ports, Shipping and Waterways, Ministry of Railways etc. Further Port/Airport Health Officers at International ports/ airports have been instructed to ensure strict health screening, testing of incoming international passengers and referrals of suspect/confirmed cases.
Further, the Union Ministry of Health & Family Welfare is in regular interaction with all States/UTs through formal communication as well as through video conferencing. States/UTs have been urged to undertake the following activities:
- Strict monitoring of International travelers in the community.
- Contact tracing of positive individuals & follow up for 14 days.
- Genome sequencing of positive samples through INSACOG Labs in a prompt manner.
- Continued monitoring of areas where clusters of positive cases emerge.
- Further strengthening of COVID-19 testing infrastructure and ensuring early identification of cases through adequate testing across the States.
- Ensure preparedness of health infrastructure (availability of ICU, Oxygen supported beds, ventilators, etc.) and upgrade health infrastructure under ECRP-II including in rural areas and for pediatric cases.
- Commissioning all PSA plants, ensuring sufficient logistics, drugs etc.
- Ensure rapid COVID-19 vaccine coverage.
- Ensuring adherence to COVID Appropriate Behaviour.
The laboratory network is continuously being strengthened progressively in the last two years both in terms of testing infrastructure as well as diagnostics. As of 1st Jan 2022, a total of 1364 government laboratories and 1753 Private Laboratories are conducting COVID-19 Testing. At present India is testing around 11-12 lakh samples a day.
A three-tier arrangement of health facilities was created for appropriate management of COVID-19 cases, [(i) COVID Care Center with isolation beds for mild or pre-symptomatic cases; (ii) Dedicated COVID Health Centre (DCHC) with oxygen supported isolation beds for moderate cases and (iii) Dedicated COVID Hospital (DCH) with ICU beds for severe cases] has been implemented. Tertiary care hospitals under ESIC, Defence, Railways, paramilitary forces, Steel Ministry etc. have been leveraged for case management.
As on 17th December 2021, there are a total of 23,680 COVID treatment facilities with 18,12,017 dedicated isolation beds (including 4,94,720 oxygen supported isolation beds) and 1,39,423 ICU beds (including 65,397 ventilator beds).
The Union Ministry of Health and Family Welfare in May 2021 issued the National Policy for Admission of COVID patients to various categories of COVID facilities with the aim to ensure prompt, effective and comprehensive treatment of patients suffering from COVID-19. As per the policy, hospitals under the Central government, State Governments and Union Territory administration including private hospitals (in States and Union Territories) managing COVID Patients shall ensure the following:
- Requirement of a positive test for COVID-19 virus is not mandatory for admission to a COVID health facility. A suspect case shall be admitted to the suspect ward of CCC, DCHC or DHC as the case may be.
- No patient will be refused services on any count. This includes medications such as oxygen or essential drugs even if the patient belongs to a different city.
- No patient shall be refused admission on the ground that he/she is not able to produce a valid identity card that does not belong to the city where the hospital is located.
- Admissions to hospitals must be based on need. It should be ensured that beds are not occupied by persons who do not need hospitalization. Further, the discharge should be strictly in accordance with the revised discharge policy available at https://www.mohfw.gov.in/pdf/ReviseddischargePolicyforCOVID19.pdf
Guidelines on Clinical management of COVID-19 continue to be updated with emerging scientific evidence. The treatment protocol for adults was last updated on 24th May 2021 and has been widely circulated. The mainstay of treatment is supplemental oxygen and other supporting therapy. No specific antivirals have been proven effective. However, drugs like Ivermectin, Hydroxychloroquine, inhalational Budesonide, Dexamethasone, Methylprednisolone and Low Molecular Weight Heparin have been recommended. In addition, provisions for Investigational Therapies have also been made using Remdesivir, and Tocilizumab for defined sub-group of patients under medical supervision.
Guidelines for management of COVID-19 in children were also updated on 18th June 2021. The guideline covers guidance on management of acute presentation of COVID-19 as well as Multisystem Inflammatory Syndrome (MIS-C) in children and adolescents found temporally related to COVID-19.
Guidelines and checklists on prevention and clinical management of Mucormycosis have also been formalized and disseminated to all States/UTs.
AIIMS, Delhi and similarly placed institutions of the States are designated Centers of Excellence for wider dissemination of latest advancements in COVID management. Telemedicine services using ‘e-sanjeevani’ for tele-consultation is one among the best practices during COVID times.
To study more about COVID sequelae, follow up clinics have been established in AIIMS and other Central Government hospitals. A Comprehensive Guidelines for Management of Post-Covid Sequelae was also issued on 21st October 2021 covering post-COVID complications affecting respiratory, cardiovascular, gastroenterological, nephrological and neurological systems.
States are being supported in terms of supply of logistics including PPE kits, N-95 masks, drugs, ventilators, oxygen cylinders, oxygen concentrators etc. States are also being supported in terms of installation of Oxygen concentrator plants/ PSA (Pressure Swing Adsorption plants) plants. Till 17th December 2021, all 1225 PSA plants have been commissioned.
In order to extend on-ground support to the State and District Health Authorities, Central multi-disciplinary teams are also being deployed to States from where upsurge of cases has been reported. Till date 173 such teams have been deployed by the Ministry of Health & FW to 33 States/UTs.
India made a remarkable turnaround to the Covid pandemic with tremendous efforts matching the challenge. By the end of October 2021, India emerged as the first country to administer 100 crore doses of vaccines to its citizens.
In terms of financial support to States, During the FY 2020-21, funds to the tune of Rs.8257.88 crore have been released to States/UTs towards the India COVID-19 Emergency Response and Health System Preparedness Package.
In addition, ‘India COVID-19 Emergency Response & Health System Preparedness Package: Phase-II’ has also been approved by the Cabinet with Rs 23,123 crores (with Rs. 15,000 Cr as Central Component & Rs 8,123 Cr as State component) and is being implemented from 1st July 2021. This includes support to State/UT level for ramping up Health Infrastructure including those in rural, tribal and peri-urban areas closer to the community, providing support for procurement of drugs and diagnostics to enhance service delivery at district and sub district levels for management of COVID-19 cases (including pediatric care) and for maintaining a buffer of drugs, support for IT Interventions such as implementation of Hospital Management Information System and expanding access to tele-consultations in all districts, and support for capacity building and training for all aspects of management of COVID-19.
Government of India through National Disaster Management Authority (NDMA) has issued ‘Guidelines to provide for ex-gratia assistance to kin of the deceased by COVID-19’. NDMA has recommended an amount of Rs. 50,000/- per deceased person including those involved in relief operations or associated in preparedness activities, subject to cause of death being certified as COVID-19. The ex-gratia assistance shall be provided by States from State Disaster Response Funds.
With the intent to develop long term capacities in preparedness for future surges of COVID-19 and other public health emergencies, PM Ayushman Bharat Health Infrastructure Mission (PM-ABHIM) has been approved with an outlay of Rs. 64,180 crores over 6 years. The PM-ABHIM envisages increased investments in public health and other health reforms to safeguard against future resurgences of COVID-19, if any, and future public health emergencies by:
- Strengthening of Health and Wellness Centers in villages and cities for early detection of diseases
- Addition of new critical care-related beds at district level hospitals.
- Operationalization of Regional National Centers for Disease Control (NCDC).
- Establishment of metropolitan units in urban areas and BSL-III labs across the country to strengthen the laboratory network.
- Strengthening of existing Viral Diagnostic and Research Labs (VRDLs) and creation of new National institutes of Virology (NIVs) and a National Institute for One Health through ICMR.
- Strengthening of Public Health Units at international Points of Entry (PoEs)
The Government of India will continue to maintain a close watch over the evolving pandemic.
2. Ayushman Bharat:
- Comprehensive Primary Health Care (CPHC) through Ayushman Bharat Health and Wellness Centres (AB-HWCs) – Ayushman Bharat aims to holistically address health (covering preventive, promotive, curative, rehabilitative and palliative care), at primary, secondary and tertiary level by adopting a continuum of care approach. In the lifetime of an individual, the primary healthcare services cater to 80-90% of the healthcare needs for improved healthcare outcomes and quality of life of the population.
- The Primary Health Care team ensures that community outreach and population enumeration are done for individuals in their catchment area and screened for communicable diseases and non-communicable diseases for early detection and timely referral for accurate diagnosis. The team further ensures that treatment adherence and follow-up care are provided to the patients in the community. These centres are aimed at delivering primary healthcare services closer to the people and be the first point of contact for healthcare provisioning and referral for secondary and tertiary care. Thus, the essential health services along with the provisioning of essential medicines and diagnostics are provided closer to the community through these centres, as a step towards building stronger and resilient primary healthcare systems which cater to the healthcare needs of the population.
- Ayushman Bharat comprises of two components:
- The first component pertains to creation of 1,50,000 Health and Wellness Centres (AB-HWCs) by upgrading the Sub Health Centres (SHCs) and rural and urban Primary Health Centres (PHCs), in both urban and rural areas, to bring health care closer to the community. These centres aim to provide Comprehensive Primary Health Care (CPHC), by expanding and strengthening the existing Reproductive & Child Health (RCH) and Communicable Diseases services and by including services related to Non-Communicable Diseases (common NCDs such as, Hypertension, Diabetes and three common cancers of Oral, Breast and Cervix) and incrementally adding primary healthcare services for mental health, ENT, Ophthalmology, Oral health, Geriatric and Palliative care and Trauma care as well as health promotion and wellness activities like yoga. A few States/UTs have already started rolling out these additional packages in a phased manner.
- The second component is the Ayushman Bharat-Pradhan Mantri Jan ArogyaYojana (AB-PMJAY). Under Ayushman Bharat – Pradhan Mantri Jan Arogya Yojana (AB-PMJAY), around 10.74 crore poor and vulnerable families identified as per Socio-Economic Caste Census are entitled for health cover of Rs. 5.00 lakh per family per year for secondary and tertiary care hospitalization. As of 01.12.2021, 33 States/Union Territories are implementing the scheme and over 2.50 crore hospitalizations amounting to approx. Rs. 28,978.32 crore have been authorized under the scheme. As on 14.11.2021, a total of 2.92 lakh hospital admissions worth over Rs. 644.5 crore have been authorized under the inter-State portability feature. Also, so far, 17.21 crore e-cards (including cards issued by the State Governments) have been issued under the Scheme for facilitating easy availing of benefits.
2.1 a. Status update on AB-HWCs:
- Operational Guidelines and training modules for MO, SNs, CHOs MPWs and ASHAs have been developed and shared with the States/UTs for rollout of expanded packages of services. These guidelines and training modules have been developed in consultation with the States/UTs incorporating the experiences of the States/UTs which have already rolled out the expanded services.
- App version of the AB-HWC portal was also launched by the Honourable HFM on the 12th of July to enable geo-tagging the location of these AB-HWCs and entering the daily service delivery parameters by the frontline healthcare workers.
- A ‘Fit Health Worker’ Campaign was also launched at these centres to enable the screening and early detection of non-communicable diseases in the Frontline-Health Care Workers. This enabled the screening of more than 13 lakhs in 537 districts till 20th December 2021 to enable them to take preventive, promotive and curative measures and also caution them towards their risk categorization towards COVID-19 as these Frontline Workers (FLWs) were not only involved in ensuring essential services at these centres but also played a crucial role in community-based surveillance and pandemic outbreak management related activities in the community.
- These centres also conduct various wellness-related activities like Yoga, Zumba, Meditation, etc., which not only enable improved physical health but also mental wellbeing of the community. It is envisaged that these centres will not only be the point of delivery for healthcare services, but at the same time enable the community to take health in their own hands. This is in addition to the 39 Health Calendar Days which are being observed at these HCFs focussing on different health promotion activities.
- In coordination with School Education Department, School Health and Wellness Ambassadors Initiative has been launched to train two teachers per school as Ambassadors on the preventive and promotive healthcare and it is planned to implement in more than 200 districts in the coming year
- Similarly, all States/UTs have started training for ‘Eat Right’ and ‘Eat Safe’ modules to the primary healthcare team at these functional AB-HWCs.
- Regional reviews of all the States and UTs are being organized virtually at the national level to understand the implementation challenges in expanding the roll-out during the COVID-19 pandemic.
- A two-day national workshop of CP-CPHC nodal officers was conducted to understand the challenges related to community processes and comprehensive primary healthcare. The best practices adopted by the States /UTs to ensure the continuity of healthcare services were also showcased and disseminated to other States/UTs for cross-learning.
2.1 b. Achievement and Service Delivery at AB-HWCs:
- So far, approvals for around 1,52,130 Ayushman Bharat-Health & Wellness Centres have been accorded to the States/UTs (except Delhi) and as reported by the States/UTs on the AB-HWC Portal, 81,518 Health & Wellness Centres have been operationalized till 20th December, 2021 which includes 55,458 SHC level AB-HWCs, 21,894 PHC level AB-HWCs and 4166 UPHC level AB–HWCs.
- As per the data update done by the States/UTs in HWC Portal, till date, more than 15 crore screenings have been done for hypertension and around 12.72 crore screenings done for diabetes at these AB-HWCs. Similarly, these functional AB-HWCs have done more than 8.23 crore screenings for oral cancer, more than 2.77 crore screenings for cervical cancer in women and more than 4.10 crore screenings for breast cancer in women.
- Further, as on 20-12-2021, a total of 92.18 lakhs Yoga/wellness Sessions have been conducted in operational AB-HWCs.
- Primary healthcare team at the Sub Health Centre level AB-HWCs is headed by Community Health Officers (CHO) – who is a BSc/GNM Nurse or an Ayurveda Practitioner trained in primary care and public health skills and certified in a six months Certificate Programme in Community Health or Graduate from Integrated nursing curriculum and other members of the team being, Multi-Purpose Workers (Male and Female) and Accredited Social Health Activists (ASHAs). The training programme is being carried out with support from IGNOU and State specific public health universities.
- The screening, prevention and management of chronic illnesses including NCDs, TB and Leprosy have been introduced as part of comprehensive primary healthcare at AB-HWCs. To roll out these services, training and skill upgradation of the primary health team in all the functional AB-HWCs on NCDs and use of IT application was undertaken.
- To promote wellness and healthy lifestyle, orientation of the public on wellness activities for lifestyle modification like increased physical activity (cyclathons and marathons), eating RIGHT and eat-SAFE, cessation of tobacco and drugs, meditation, laughter clubs, open gyms, etc are being undertaken in states. Besides, Yoga Sessions are carried out at these centres on a regular basis.
- The telemedicine guidelines have also been provided to the States to initiate specialist consultations from the PHCs to the Hub Hospitals. So far, 56,927 AB-HWCs have initiated the tele-consultations.
2.2 Human Resources:
NHM has attempted to fill the gaps in human resources by providing nearly 2.74 lakh additional health human resources to the States including 13,074 GDMOs, 3,376 Specialists, 73,847 Staff Nurses, 85,834 ANMs, 48,332 Paramedics, 439 Public Health Managers and 17,086 Programme Management staffs appointed on contractual basis. Apart from providing financial support for hiring human resources, NHM has also focused on multi-skilling of human resources and providing technical support for human resources in the health sector in the form of technical guidance and training. NHM also supports co-location of AYUSH services in health facilities such as PHCs, CHCs and DHs. A total of 27,737 AYUSH doctors and 4564 have been deployed in the states with NHM funding support.
2.3 Mainstreaming of AYUSH:
Mainstreaming of AYUSH has been taken up by allocating AYUSH services in 7,452 PHCs, 2,811 CHCs, 487 DHs, 4,022 health facilities above SC but below block level and 456 health facilities other than CHC at or above block level but below district level.
2.4 Infrastructure:
Up to 33% of NHM funds in High Focus states can be used for infrastructure development. Details of new construction/renovation as on 30.06.2021 undertaken across the country under NHM are as follows:
|
Facility |
New Construction |
Renovation/Upgradation |
|||
|
Sanctioned |
Completed |
Sanctioned |
Completed |
||
|
SC |
35805 |
22073 |
26125 |
19464 |
|
|
PHC |
2889 |
2447 |
16783 |
14582 |
|
|
CHC |
604 |
530 |
7636 |
14582 |
|
|
SDH |
251 |
174 |
1317 |
1145 |
|
|
DH |
175 |
156 |
3311 |
2854 |
|
|
Others* |
1337 |
802 |
3310 |
1365 |
|
|
Total |
41061 |
26182 |
58282 |
48072 |
|
|
|
|
|
|
|
|
* These facilities are above SCs but below block level.
2.5 National Ambulance Services (NAS):
As on date, 35 States/UTs have the facility where people can dial 108 or 102 telephone numbers for calling an ambulance. Dial 108 is predominantly an emergency response system, primarily designed to attend to patients of critical care, trauma and accident victims etc. Dial 102 services essentially consist of basic patient transport aimed to cater the needs of pregnant women and children though other categories are also taking benefit and are not excluded. JSSK entitlements e.g. free transport from home to facility, inter facility transfer in case of referral and drop back for mother and children are the key focus of 102 service. This service can be accessed through a toll-free call to a Call Centre. As of June 2021, 35 States/UTs have the facility where people can dial 108 or 102telephone numbers for calling an ambulance. Presently 11,879 Dial-108 and 10,716 (Dial-102/104) Emergency Response Service Vehicles are supported under NHM, besides 5,124empaneled vehicles for transportation of patients, particularly pregnant women and sick infants from home to public health facilities and back.
2.6 National Mobile Medical Units (NMMUs):
To increase visibility, awareness and accountability, all Mobile Medical Units have been positioned as “National Mobile Medical Unit Service” with universal colour and design. As on June 2021, States/UTs have 1,634 mobile medical units which includes mobile medical units, mobile health units, mobile medical/health vans, boat clinics, eye vans/mobile ophthalmic units, dental vans under NRHM and NUHM.
2.7 Free Drugs Service Initiative:
Under this Initiative, substantial funding is being given to States for provision of free drugs and setting up of systems for drug procurement, quality assurance, IT based supply chain management system, training and grievance redressal etc. Detailed Operational Guidelines for NHM-Free Drugs Service Initiative were developed and released to the States on 2nd July, 2015.
All the States and UTs have notified policy to provide essential drugs free in health facilities, have facility wise EDL and have centralized procurement through a corporation/procurement body. Drugs procurement, quality system and distribution has been streamlined through IT enabled Drugs Distribution Management Systems in 33 States/UTs, 31 States/UTs have NABL accredited labs to ensure quality of drugs provided, 18 States/UTs have prescription audit mechanism and 17 States have established call center based grievance redressal mechanism with dedicated toll free number.
2.8 Free Diagnostics Service Initiative:
To address the need of accessible and quality diagnostics in public health facilities, the Ministry of Health and Family Welfare launched Operational Guidelines on Free Diagnostics Service Initiative in consultation with experts and the States officials and disseminated among States/UTs in July 2015. The government envisaged that this health intervention will reduce both direct costs and Out of Pocket expenditure. This guideline supports States/UTs to provide essential diagnostics-Laboratory services and Radiology services (Tele radiology and CT scan Services) at their public health facilities.
The second edition of Free Diagnostics Initiative has been released which provides a broader view of the expanded basket of laboratory services envisaged under National Health Mission. The revised guidelines on FDSI recommend an expanded basket of tests of 14/63/97/111/134 at SC/PHC/CHC/SDH/DH respectively. A dissemination workshop was organised by NHSRC to guide States/UTs for implementation of the guidelines.
As on 1st December 2021, free diagnostics laboratory services have been implemented in 33 States/UTs. (In 11 States/UTs it is implemented in PPP mode and in 22 States/UTs it is in In-house mode). Free Diagnostics CT Scan services have been implemented in 23 States/UTs (In 13 States/UTs it is implemented in PPP mode and in 10 States/UTs it is in In-house mode). Free Teleradiology Services have been implemented in 13 States/UTs in PPP mode.
2.9 Biomedical Equipment Maintenance and Management Programme:
Bio-medical Equipment Management and Maintenance Programme (BMMP) was launched in 2015 by the Ministry of Health and Family Welfare, Government of India with the goal of equipment upkeep time of 95% for DHs, 90% for CHCs and 80% for PHCs. The program provides support to state governments to outsource medical equipment maintenance comprehensively for all facilities so as to improve the functionality and life of equipment, simultaneously improving healthcare services in public health facilities- reducing cost of care and improving the quality of care. This program helped in converting pending dysfunctional equipment to functional in States/UT following BMMP.
Biomedical Equipment Management and Maintenance Program technical guidance document for in-house support and monitoring of public private partnerships is circulated to States/UTs.
As on 1st December 2021, BMMP has been implemented in 30 States/UTs (In 23 States/UTs it is implemented in PPP mode and 7 States/UTs it is in In-House mode).
2.10 Community Participation:
- Accredited Social Health Workers: There are 9.83 lakh ASHAs are in position across the country in rural and urban areas(except Goa and Chandigarh) under the NHM who act as a link between the community and the public health systemThe Union Cabinet has approved increase in amount of routine and recurring incentives under National Health Mission for ASHAs that will now enable ASHAs to get at least Rs 2000/- per month against Rs 1000 earlier. The cabinet has also approved a proposal to cover all ASHAs and ASHA facilitators meeting eligibility criteria under Pradhan Mantri Jeevan Jyoti Bima Yojana and Pradhan Mantri Suraksha Bima Yojana which would be fully funded by Government of India.
Under the Pradhan Mantri Shram Yogi Maandhan (PM-SYM) PM-SYM which has been rolled out nation-wide on 15th February, 2019 and is a voluntary contributory pension scheme to ensure old age protection for unorganized workers between 18 and 40 years of age with a monthly income of Rs.15000/- or below ,the ASHAs and ASHA Facilitators in the specified age group are invariably eligible under the scheme. The scheme requires self-certification, 50% of the monthly contribution for the pension scheme will be contributed by the Central Government while the remaining 50% is to be contributed by the beneficiary. The amount varies with the age of the beneficiary and it will be auto-deducted from the bank account of the beneficiary. The Ministry of Labour and Employment has made the provision of bulk enrolment facility as well through CSC-SPV. The beneficiaries under the scheme will receive a minimum assured pension of Rs 3000/- per month after attaining the age of 60 years.
- VHSNCs: 5.55 lakh Village Health Sanitation and Nutrition Committees (VHSNCs) at village level have been constituted across the country to facilitate village level healthcare planning. 1.30 crore Village Health Sanitation & Nutrition Days (VHSNDs) were held during FY 20-21.
- Untied Grants to Sub-Centres (SCs): At the Village Level, the Village Health, Sanitation and Nutrition Committee (VHSNC) monitors the services provided by the Aanganwadi Worker, the ASHA and the sub-centre. These Committees are envisaged to function under the ambit of the Panchayati Raj Institution with adequate representation from women and weaker sections of the society. The VHSNC acts as a subcommittee or statutory body of the Gram Panchayat. The same institutional mechanism is also mandated in urban areas. VHSNCs are provided an untied fund of Rs 10,000 on annual basis which are topped up based on expenditure of previous year. More than 5.55 lakh VHSNCs have been set up across the country till June2021. The capacity building of VHSNC members with regards to their roles and responsibilities for maintaining the health status of the village is being done in many states.
2.11 24 X 7 Services and First Referral facilities:
As of June 2021, 10,951 PHCs have been made 24×7 PHCs and 3001 facilities (including 690 DH, 763 SDH and 1548 CHCs & other levels) have been operationalized as First Referral Units (FRUs) under NHM. To ensure service provision for Maternal and Child Health, 24×7 services at the PHCs have been made available. 10,430 PHCs have been made 24×7 PHCs and 3346 facilities (including 653 DH, 862 SDH and 1831 CHCs & other levels) have been designated as First Referral Units (FRUs) under NHM.
2.12 Mera Aspataal:
Recognizing the need to capture the voice of patients for enhanced patient experience and continued learning, India launched its own centralized IT platform i.e. ‘Mera-Aspataal’/ ‘My Hospital’. ‘MeraAspataal’ is a patient feedback system which was launched in September 2016 with a mandate to integrate Central Government Hospitals (CGHs) & District Hospitals (DHs). It has now been extended upto CHC, Rural & Urban Primary Health Centre and private medical colleges and is currently functional in 34 States/UTs. As of now, on 7th December’21, 9446 government health facilities and 736 non-governmental health facilities are integrated with Mera-Aspataal in 34 States and UTs.
2.13 Kayakalp:
As part of contribution towards the Swachh Bharat Abhiyaan launched by the Prime Minister on 2nd October 2014, ‘Kayakalp’ award scheme was launched by the Ministry of Health & Family Welfare in 2015 to promote practice of cleanliness, hygiene & sanitation, and controlling the hospital acquired infection in urban & rural public health facilities. Facilities which outshine and excel against the predefined criteria are awarded. Incentivization amount ranges from Rs. 50.00 Lakh for winner DH to Rs. 25,000 as commendation award for HWC.
From FY 2015-16 to FY 2020-21 the number of Kayakalp Awardee facilities have increased from 100 facilities to 12431 facilities in 2020-21 (DHs- 456, SDH/CHCs- 2473, PHCs- 6281, UPHCs- 1270, UCHCs-19 & HWCs-1932) .
2.14 Swachh Swasth Sarvatra:
- SwachhSwasthSarvatra is a joint initiative of the Ministry of Health & Family Welfare and Ministry of Drinking Water and Sanitation (Now Ministry of Jal Shakti) to achieve better health outcomes through improved sanitation and increase awareness on healthy lifestyles.
- This initiative was launched in December 2016, to build on and leverage the achievements of the two programmes – Swachh Bharat Mission (SBM) and Kayakalp – of the Ministry of Drinking Water and Sanitation and Ministry of Health and Family Welfare, respectively.
- Based on its result and success in rural areas, ‘’Swachh Swasth Sarvatra’’ was implemented in urban areas in Year 2019. In urban areas it is implemented through joint initiatives of the Ministry of Housing and Urban Affairs and Ministry of Health and Family welfare.
Objectives of the program: –
- Enabling Gram Panchayat, cities and wards, where Kayakalp awardee PHCs/UPHCs are located, in sustaining ODF and promoting healthy behaviour.
- Strengthening CHC/UCHCs/UPHCs in ODF blocks/Wards/Cities to achieve a high level of cleanliness to meet Kayakalp standards through a support of Rs 10.0 L for CHCs/UCHCs and Rs 50K for UPHCs under NHM.
- Build capacity through training in Water, Sanitation and Hygiene (WASH) to nominees from such CHCs and PHCs.
Progress under Swachh Swasth Sarvatra:-
- Under this initiative funds of Rs 1948 lakhs were approved as a one time grant up to Rs 10 Lakhs for 192 CHCs & Rs 50,000 for 356 UPHCs across the country to achieve a minimum 70% benchmark of Kayakalp in the year 2021-22.
- Number of health facilities achieving the Kayakalp awards criterion is increasing every year with this programme. Number of CHCs that won Kayakalp awards increased from 323 CHCs in FY 2016-17 to 2004 CHCs in 2020-21. Number of UPHCs that won Kayakalp awards increased from 556 in 2018-19 to 1270 in 2021-22.
2.15 National Quality Assurance Programme:
Quality in delivered health care services is important for improving the health status of the population. It enhances accessibility, increases efficiency, strengthens clinical effectiveness and improves user satisfaction. With the aim of improving quality of care, the Ministry of Health and Family Welfare launched the National Quality Assurance Standards (NQAS) for District Hospitals in 2013 and subsequently for other health facilities. These standards are internally accredited by ISQua (International Society for Quality in Healthcare).
These standards are also recognized by IRDA and NHA. At present, total number of NQAS certified facilities till 7th December 2021 is 1316, out of which 620 Public Health Facilities have achieved National Quality Certification in current calendar year (January to December 2021) , summary for the same is as follows:
|
Category |
Total National Certified Facilities (2016 – 2021) |
National Certified Facilities from January 2021 to December 2021 (Calendar Year wise) |
|
DH |
148 |
32 |
|
SDH |
41 |
2 |
|
CHC |
98 |
24 |
|
PHC |
931 |
510 |
|
U-PHC |
98 |
52 |
|
Total |
1316 |
620 |
Apart from this,
To provide Comprehensive Primary Health Care (CPHC) through Health and Wellness Centres (AB-HWCs), Essential Medicines List (EML) for SHC and PHC have been finalized. To strengthen the Free Drugs Service Initiative (FDSI), Indian Public Health Standards (IPHS) guidelines are being revised for Sub-Centres, Primary Health Centres (PHCs), Community Health Centres (CHCs), Sub District Hospitals (SDHs), District Hospitals (DHs) and also being developed for Urban Health (U-PHC). Essential Drugs are the integral part of IPHS guidelines to support the healthcare system in achieving sustainable Development Goal.
MusQan: The nation has made remarkable progress in the last two decades to reduce the number of child deaths, but the neonatal mortality rate has declined at a slower pace. A large proportion of newborn deaths are preventable. Providing quality paediatric care services through the public health facilities is one of the mandates of the National Health Mission.
The National Quality Assurance Standards (NQAS) are already implemented in the states and UTs to ensure delivery of quality care within the facilities.
Under the ambit of the NQAS, to ensure delivery of bench-marked quality and safe care to children at Public Health, a new initiative named “MusQan” was launched by the Hon’ble Health Minister on 17 September 2021. The objective of MusQan is to reduce child mortality and morbidity and improve nutrition status, growth, and early childhood development of young children through strengthening clinical protocols and management processes and provision of respectful and dignified care to newborns and children.
Recently, a national dissemination workshop was conducted on 3rd December 2021 to orient the states and UTs about the key initiative and start preparing an implementation plan at the state, district and the facility level.
2.16 National Urban Health Mission (NUHM)
National Urban Health Mission (NUHM) was approved on 1st May, 2013 as a sub-mission under an overarching National Health Mission (NHM), NRHM being the other sub-mission. NUHM envisages strengthening the primary health care delivery systems in urban areas and for providing equitable and quality primary health care services to the urban population with special focus on slum dwellers and vulnerable population. It also seeks to decongest secondary and tertiary health care facilities (District Hospitals/Sub-District Hospitals/Community Health Centre) by providing robust comprehensive Primary health care services in urban areas.
NUHM covers all cities and towns with more than 50,000 populations and district headquarters and State headquarters with more than 30,000 population. The remaining cities/ towns continue to be covered under National Rural Health Mission (NRHM). As part of Ayushman Bharat, the existing UPHCs are being strengthened as Health & Wellness Centres (HWCs) to provide preventive, promotive and curative services in cities closer to the communities.
Under NUHM, the Centre-State funding pattern is 60:40 for all the states w.e.f. FY 2015-16, except all North-Eastern states and other hilly States viz. Jammu & Kashmir, Himachal Pradesh and Uttarakhand, for which the Centre-State funding pattern is 90:10. In the case of UTs, from FY 2017-18, the funding pattern of UT of Delhi and Puducherry has been revised to 60:40 and rest of the UTs without legislature are fully funded by the Central Government.
Implementation of NUHM is through the State Health Department or the Urban Local Bodies (ULBs). In seven metropolitan cities, viz., Mumbai, New Delhi, Chennai, Kolkata, Hyderabad, Bengaluru and Ahmedabad the implementation is through the ULBs. For the other cities, the State Health Department decides whether the NUHM is to be implemented through them or the other urban local bodies. So far, 1162 cities have been covered under NUHM in 35 States/UTs.
Physical Progress:
The programme is being implemented in the States/UTs for more than 7 years period and accounts for presence of augmented infrastructure and human resources dedicated towards urban areas. According to the 2nd Quarterly Progress Report (QPR) i.e. for period July-Sept, 2021 submitted by the States/UTs, the information regarding progress of activities approved under NUHM is as follows: –
-
- 3135 Medical Officers in-position against 4379 approved
- 238 Specialists in-position against 537 approved
- 6537 Staff Nurse in-position against 10863 approved
-
- 14113 ANMs in-position against 19557 approved
- 2898 Pharmacist in-position against 4142 approved
- 3265 Lab Technician in-position against 4269 approved
- 436 Public Health Managers in-position against 752 approved
- 1180 Programme Management staff in-position at State/District/City level against 1474 approved
- So far, 1162 cities/ towns covered under NUHM
- 5501 existing facilities approved for strengthening as Urban PHCs
- 897 new U-PHCs construction approved
- 90 new U-CHCs construction approved
- 101 Mobile Health Units approved
- 643 Health Kiosks approved
For slum habitations
- 68712 ASHAs engaged against 81349 approved. (One ASHA covers 200 to 500 households)
- 76267 Mahila Arogaya Samiti (MAS) formed against 98615 approved. (One MAS covers 50- 100 households)
Kayakalp and Swachh Swasth Sarvatra (SSS) have been expanded to cover urban areas also and U-PHCs have been awarded Kayakalp awards. Out of 35 States/UTs, 33 States and UTs declared Kayakalp awards for FY 2021-22, and 1198 UPHCs and 16 UCHCs have won Kayakalp awards.
To ensure delivery of Comprehensive Primary Health Care (CPHC) services under the Health and Wellness Centre component of Ayushman Bharat, the existing UPHCs are being strengthened as Health and Wellness Centres (HWCs). Support for training of PHC staff (Medical Officers, Staff Nurses, Pharmacist, and Lab Technicians), necessary IT infrastructure and the resources required for upgrading laboratory and diagnostics for expanded ranges of services is being provided to the States. So far 4203 HWCs have been operationalized in urban areas as of 29.11.2021.
Financial Progress:
Since the launch of NUHM in FY 2013-14 till the 7th December, 2021, funds to the tune of Rs. 8788.48 Crore and Rs.7040.11 Crore have been allocated and released respectively to the States/ UTs for implementation of the programme activities.
2.17 NHM Best Practices and Innovations, 2020: Good Replicable and Innovative practices
The National Summit on Good and Replicable Practices and Innovations in Public Healthcare Systems in India is an institutional mechanism for sharing of innovations. The 7th National summit was held online in webinar mode due to Covid-19 conditions. The summit was attended by the Principal Secretary/ Secretary of Health from all the states and UTs along with Mission directors and Program officers. On 13th Dec, 2021, Hon’ble Union Minister of Health & Family Welfare released the Coffee Table Book in Ebook format documenting all practices presented in the 7th National Summit. It captures 47 best practices and innovations, out of which 23 are oral and 24 are poster presentations. They span programmatic areas ranging from health systems, maternal and newborn health, family planning, chronic and other communicable diseases, non-communicable diseases, mental health, and e-health.
2.18 Pradhan Mantri Ayushman Bharat Health Infrastructure Mission (PM-ABHIM)
Pradhan Mantri Atmanirbhar Swasth Bharat Yojana scheme (now renamed as Pradhan Mantri Ayushman Bharat Health Infrastructure Mission) with an outlay of about Rs. 64,180 Cr over till FY 2025-26 was launched by Hon’ble Prime Minister on 25th October, 2021. This is the largest pan-India scheme for strengthening healthcare infrastructure across the country.
The measures under the scheme focus on developing capacities of health systems and institutions across the continuum of care at all levels viz. primary, secondary and tertiary and on preparing health systems in responding effectively to the current and future pandemics/disasters.
The Pradhan Mantri Ayushman Bharat Health Infrastructure Mission targets to build an IT enabled disease surveillance system by developing a network of surveillance laboratories at block, district, regional and national levels, in Metropolitan areas & strengthening health units at the Points of Entry, for effectively detecting, investigating, preventing, and combating Public Health Emergencies and Disease Outbreaks.
Increased investments are also targeted to support research on COVID-19 and other infectious diseases, including biomedical research to generate evidence to inform short-term and medium-term response to COVID-19 like pandemics and to develop core capacity to deliver the One Health Approach to prevent, detect, and respond to infectious disease outbreaks in animals and humans.
The main interventions under the ‘Pradhan Mantri Ayushman Bharat Health Infrastructure Mission’ scheme to be achieved by FY 2025-26 are:
Centrally Sponsored Components:
- Support for 17,788 rural Health and Wellness Centres in 10 High Focus States. Support for other States/UTs under XV Finance Commission Health Sector Grants and NHM.
- Establishing 11,024 urban Health and Wellness Centres in all the States.
- 3382 Block Public Health Units in11 High Focus states. Support for other States/UTs under XV Finance Commission Health Sector Grants and NHM.
- Setting up of Integrated Public Health Labs in all districts.
- Establishing Critical Care Hospital Blocks in all districts with population more than 5 lakhs.
Central Sector Components:
- 12 Central Institutions as training and mentoring sites with 150 bedded Critical Care Hospital Blocks.
- Strengthening of the National Centre for Disease Control (NCDC), 5 New Regional NCDCs and 20 metropolitan health surveillance units;
- Expansion of the Integrated Health Information Portal to all States/UTs to connect all public health labs;
- Operationalisation of 17 new Public Health Units and strengthening of 33 existing Public Health Units at Points of Entry, that is at 32 Airports, 11 Seaports and 7 land crossings;
- Setting up of 15 Health Emergency Operation Centres and 2 container based mobile hospitals; and
Setting up of a national institution for One Health, 4 New National Institutes for Virology, a Regional Research Platform for WHO South East Asia Region and 9 Biosafety Level III laboratories.
3.Reproductive, Maternal, Newborn, Child, Adolescent Health Plus Nutrition (RMNCAH+N)
3.1 Immunization
- Pneumococcal Conjugate Vaccine (PCV) nationwide expansion: PCV was launched in Phased manner in May 2017 and till FY 2019-20, it was available in five states in the country. In FY 2020-21, in line with the Budget announcement 2021-22, PCV has been expanded nationwide and is now available across all States/UTs.
- % increase in India’s FIC as per NFHS-5: NFHS 5 survey report has shown 14.4 percentage points increase in Full Immunization Coverage as compared to NFHS-4.
- National COVID-19 Vaccination Programme:
On 16th January 2021, India launched the National COVID Vaccination Programme. COVID vaccination in the country commenced with vaccination to all Health Care Workers. The Vaccination Programme is being guided by immaculate planning based on a regular review of scientific and global test practices by National Expert Group on Vaccine Administration for COVID-19 (NEGVAC).
Since the start of the COVID Vaccination drive, it has focused on taking decisions guided by science. Prioritizing our health workers, frontline workers and other vulnerable populations in a phased manner has been an excellent way to scale up the vaccination program. Now, all adults are eligible for COVID vaccination. Soon, we would be vaccinating the children too.
Under the programme, all citizens irrespective of their income status are entitled to free vaccination. While those who have the ability to pay are encouraged to use private hospital’s vaccination centres.
Three vaccines are being used in COVID Vaccination Drive, these include two made in India vaccines namely Serum Institute of India’s Covishield, Bharat Biotech’s Covaxin and Russian Sputnik V (being used only in private hospitals).
In just 9 months of the start of the COVID vaccination drive, India achieved a significant milestone of administering over 100 crore doses of COVID vaccines to its eligible adult population. India became one of the few countries to achieve this milestone.
Out of the total eligible adult population, as on 7th December 2021, over 85% citizens had received 1st dose of COVID Vaccine while over 50% eligible citizens had received 2nd dose of the vaccine.
Har Ghar Dastak
A nation-wide COVID-19 vaccination campaign Har Ghar Dastak was implemented from 3rd Nov till 31st Dec 2021 which included mobilization, awareness, vaccination campaign through reaching out to all missed out and dropped out eligible beneficiaries through House to House visit.
The campaign aimed to ensure that all eligible beneficiaries are vaccinated with 1st dose and all due beneficiaries with 2nd dose of COVID-19 vaccines. The Ministry had created and shared an operational guideline that was shared with all State/UT.
With a special focus on the low performing districts, nodal officers (Joint Secretaries) were identified for regular follow ups and visits to the assigned states.
Further, Hon’ble Health & Family Welfare Minister also held an orientation session with NGOs & CSOs from across the country on the Har Ghar Dastak campaign. He discussed how an enhanced partnership between the government and these organizations would strengthen the campaign.
Achievement / Progress under Har Ghar Dastak Campaign:
Due to these efforts and sustained efforts by all States and UT, 1st dose coverage increased by 5.3% during Har Ghar Dastak Abhiyan (data till 30th Nov, 2021).
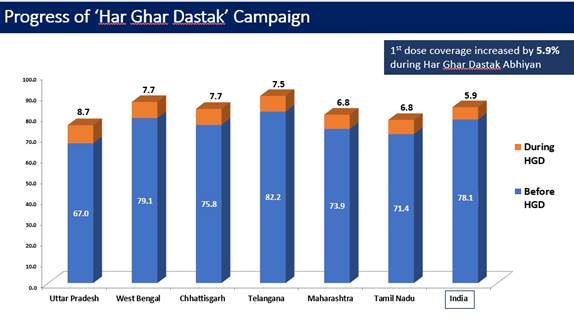
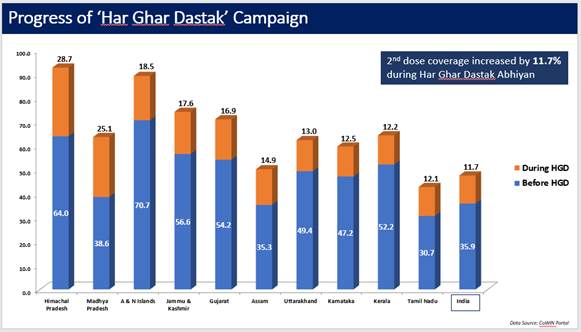
Similarly, the 2nd dose coverage increased by 11.7% during the campaign (data till 30th Nov, 2021).
Best Practices under Har Ghar Dastak Campaign:
Bihar: Ek Adhoora, Do se Poora Campaign – Mission 2nd Dose Campaign; Teeka Express; Teeka Wali Nav; and Motorbike Vaccination team.
Himachal Pradesh: Suraksha ke rang naa honge pheeke jab samay par lagenge dono teeke initiative; Bulawa toli.
Maharashtra: Committee of Sarpanch, Talathi, Gram Sevak, ASHA, AWW, Teachers for mobilization.
Manipur: Religious Leaders Appealed for vaccination through recorded messages. These messages were shared through WhatsApp.
Andhra Pradesh: Rewarding of best performing teams by District Civil Surgeon Kalaa Jathara, Dandora.
Jharkhand: Nukkad Natak, Ramp & Wall writing.
- Sustaining Routine Immunization during COVID-19 pandemic: Clear strategy & guidelines have been developed and special efforts have been made to sustain Routine Immunization, conduct National Immunization Days and Sub National Immunization Days for Polio, conduct intensified immunization drives such as Mission Indradhanush to reduce the gap in coverage and conduct surveillance for Vaccine Preventable Diseases (VPDs) during the COVID-19 pandemic.
3. Reproductive, Maternal, Newborn, Child, Adolescent Health Plus Nutrition (RMNCAH+N)
3.1 Immunization
- Pneumococcal Conjugate Vaccine (PCV) nationwide expansion: PCV was launched in phased manner in May 2017 and it was available in five States in the country till FY 2019-20. In FY 2021-22, in line with the Budget announcement 2021-2022, PCV has been expanded nationwide and is now available across all States/UTs.
- Increase in India’s Full Immunization Coverage (FIC) as per NFHS-5 (2019-21): NFHS-5 survey report has shown 14.4 percentage points increase in FIC as compared to NFHS-4.
- Sustaining Routine Immunization during COVID-19 pandemic: Strategy & guidelines have been developed and special efforts have been made to sustain Routine Immunization, conduct National Immunization Days and Sub-National Immunization Days for Polio, conduct intensified immunization drives such as Mission Indradhanush to reduce the gap in coverage and conduct surveillance for Vaccine Preventable Diseases (VPDs) during the COVID-19 pandemic.
3.2 Maternal Health
a) Key highlights of NFHS-5 (2019-21)-Maternal Health:
● 1st Trimester ANC Registration increased from 58.6% (NFHS-4) to 70% in NFHS-5
● Institutional Deliveries increased from 78.9% (NFHS-4) to 88.6% in NFHS-5
● Skilled Birth Attendant (SBA) attended deliveries increased from 81.4% (NFHS-4) to 89.4% in NFHS-5.
b) Surakshit Matritva Aashwasan (SUMAN): It aims to provide assured, dignified, respectful and quality healthcare at no cost and zero tolerance for denial of services for every woman and newborn visiting the public health facility to end all preventable maternal and newborn deaths. Till 14th December 2021, 10,010 facilities have been notified under SUMAN.
c) Maternal Perinatal Child Death Surveillance Response (MPCDSR) software was launched by the Hon’ble Union health Minister of Health & Family Welfare in September 2021. This was followed by the National ToT of the software in October 2021.
d) Midwifery Educator Training: The Government of India has taken a policy decision to roll out Midwifery Services in the country in order to improve the quality of care and ensure respectful care to pregnant women and newborns. “Guidelines on Midwifery Services in India, 2018” was released during the Partners Forum held in December 2018 at New Delhi.
● Resumption of Midwifery training: Training of Midwifery Educators (MEs) was halted due to the pandemic, which was resumed in September 2021 at NMTI in Telangana.
● Release of Scope of Practice: “Scope of Practice document for Midwifery Educators (ME) and Nurse Practitioner Midwife (NPM)” has been released in collaboration with the Indian Nursing Council (INC). It acts as a guiding document for their education, regulation and ongoing professional development.
e) Pradhan Mantri Surakshit Matritva Abhiyan (PMSMA): Since inception, more than 3.02 crore antenatal check-ups have been conducted and 25.46 lakh high risk pregnancies have been identified under PMSMA across States/ UTs till 4th December 2021.
f) LaQshya: It aims to improve the quality of care in Labour Room and Maternity Operation Theatres to ensure that pregnant women receive respectful and quality care during delivery and immediate postpartum. Till 30th November 2021, 421 Labour Rooms and 350 Maternity Operation Theatres are LaQshya certified at national level. During the FY 2021-22, 99 Labour Rooms and 79 Maternity Operation Theatres are LaQshya certified at national level.
g) Janani Suraksha Yojana (JSY): 36.38 lakhs beneficiaries received benefits under JSY during the period of April-September 2021 (Provisional data, 2021-22).
h) Ensuring Maternal health services during Covid-19 pandemic: On 19th May 2021, a webinar was conducted on ‘Ensuring Maternal Health Services in COVID-19 Pandemic’ with support from domain experts and some State’s maternal health nodal officers with the objective to re-emphasize and reinforce MoHFW’s guidance on essential maternal health services during COVID-19 pandemic and also to impart standardized and updated knowledge on management of COVID-19 during different stages of pregnancy and to disseminate good practices from the States and medical colleges.
i) Guidelines released:
● Collaborative framework for management of tuberculosis in pregnant women was released to help the States / UTs, Mission Directors and programme officers to ensure early detection and timely management of TB cases in pregnant women in India. National training has been planned for FY 2021-22.
● Standard Operating Procedures for HIV & Syphilis Screening of Pregnant Women at VHSND Sites was released to define the standard operating procedures for implementation of HIV & Syphilis screening at VHSND sites.
● Guidelines on operationalisation of maternal health services during COVID-19 pandemic’ was finalised and released in September 2021.
j) Comprehensive Abortion Care (CAC): More than 16,000 Medical Officers have been trained in CAC trainings upto December, 2021. Virtual training of trainers (ToT) on CAC has been conducted for 17 States and 328 Master Trainers have been trained till December 2021.
k) Medical Termination of Pregnancy (Amendment) Act & Rules 2021: The MTP Act recognized the importance of providing safe, affordable, accessible and legal abortion services to woman who needs to terminate a pregnancy due to certain therapeutic, eugenic, humanitarian or social grounds. The Act was amended for expanding base of beneficiaries to provide safe abortion services.
The Medical Termination of Pregnancy (Amendment) Act, 2021 was published in the Gazette on 25th March 2021 and followed by its notification for commencement on 24th September, 2021. The Rules were formed and notified for commencement on 12th October 2021.
The amended MTP Act is a step towards safety and well-being of women and will enlarge the ambit and access of women to safe and legal abortion without compromising on safety and quality of care.
The Medical Termination of Pregnancy (Amendment) Act, 2021 has introduced the following changes in The MTP Act 1971:
● Requirement of opinion of one registered medical practitioner for termination of pregnancy up to twenty weeks of gestation
● Requirement of opinion of two registered medical practitioners for termination of pregnancy of twenty to twenty-four weeks of gestation
● Enhanced the upper gestation limit from twenty to twenty-four weeks for such category of woman as may be prescribed by rules in this behalf
● Non-applicability of the provisions relating to the length of pregnancy in cases where the termination of pregnancy is necessitated by the diagnosis of any of the substantial foetal abnormalities diagnosed by a Medical Board
● Strengthening protection of privacy of a woman whose pregnancy has been terminated
● Failure of contraceptive clauses extended to women and their partner.
3.3 Child Health
a) As per the latest report of Sample Registration System (SRS) released in October 2021 by the Registrar General of India (RGI), Infant Mortality Ratio (IMR) of India has declined from 32 per 1000 live births for the year 2018 to 30 per 1000 live births for the year 2019.
27 States/ UTs namely Mizoram, Nagaland, Sikkim, Kerala, A & N Islands, Goa, Lakshadweep, Puducherry, Manipur, Delhi, D & N Haveli, Chandigarh, Tamil Nadu, Maharashtra, Daman & Diu, Punjab, Himachal Pradesh, Jammu & Kashmir including Ladakh, West Bengal, Karnataka, Tripura, Telangana, Andhra Pradesh, Gujarat, Haryana, Jharkhand, Uttarakhand have achieved National Health Policy Target (28 per 1000 live births by 2019).
b) Facility Based Newborn Care (FBNC) program: 914 Special Newborn Care Units (SNCUs) at District/ Medical College Level and 2,579 Newborn Stabilization Units (NBSUs) at the level of FRUs/ CHC levels are functional to provide services to sick and small newborns. A total of 7.53 lakhs newborns received treatment in Special Newborn Care Units (SNCUs) at District Hospitals and Medical Colleges (April-November, 2021).
c) National Newborn Week is observed from 15th to 21st November every year to reinforce the importance of newborn health as a key priority area and reiterates its commitment at the highest level. In the year 2021 also, a virtual event for the National Newborn Week was organized by MoHFW on 15th November 2021. The theme of National Newborn Week for this year is “Safety, Quality and Nurturing care – Birth Right of Every Newborn”. National Newborn Week and SAANS Campaign IEC posters were also released by MoHFW on this day for dissemination of information and for triggering behaviour change and demand generation on newborn health.
d) MusQan – Quality improvement initiative of Child Health services: The Hon’ble Union Minister of Health and Family Welfare launched “MusQan” initiative on 17th September 2021 for ensuring child friendly services in Public Health facilities on the occasion of World Patient Safety Day. The initiative will be focusing on improving the quality parameters for ensuring safety and availability of infrastructure, equipment, supplies, skilled human resources, clinical protocols, evidence based practices etc. at public health facilities. National dissemination of “MusQan – Quality improvement initiative of Child Health services” was conducted on 3rd December 2021.
e) Home Based Newborn Care (HBNC) program: A total of 98.63 lakh newborns received complete schedules of home visits by ASHAs whereas 3.6 lakhs identified sick newborns were referred to health facilities by ASHAs during the period of January-September 2021.
f) Home Based Care of Young Child (HBYC): In FY 2021-22, approval has been accorded for 604 Districts including all Aspirational Districts to implement HBYC across States/UTs except Goa. More than 1.2 crores home visits conducted to young children (3 months-15 months) by ASHAs during January-September, 2021. In addition, a supportive supervision handbook for ASHA Facilitators and ANM/MPW on HBNC and HBYC programs has been shared with all States/UTs aiming to improve on job mentoring and supervision by AF/ANM/MPW to ensure quality home visits by ASHAs.
g) Under Intensified Diarrhoea Control Fortnight (IDCF), 2021, approximately 8 crore children up to five years of age were provided with ORS and Zinc against the target of 13.37 crore children of the same age group. The data compilation for the IDCF/Diarrhoea prevention activities for the year 2021 round is in process.
h) National Deworming Day (NDD): During 12th round of NDD conducted in February 2021, around 17.75 crore children in the age group of 1-19 years had been provided Albendazole tablets against the target of 20.94 crore children of the same age group. The 13th round of NDD is being implemented in 34 States / UTs during the period of August-November, 2021.
i)Nutrition Rehabilitation Centres (NRCs): Nearly 1.04 Lakhs Severe Acute Malnutrition (SAM) children with medical complications received treatment at 1073 Nutrition Rehabilitation Centres during FY 2020-21. During FY 2021-22 (April-September 2021), 59,424 Severe Acute Malnutrition (SAM) children with medical complications received treatment at 1080 NRCs.
j) Lactation Management Centres (LMCs): As of FY 2020-21, 15 CLMCs and 3 LMUs are established in 7 States (Maharashtra, West Bengal, Goa, Gujarat, Madhya Pradesh, Tamil Nadu and Uttar Pradesh).
k) Anemia Mukt Bharat (AMB) program (April-September, 2021)
-
- 2.0 crore children of age group 6-59 months were provided 8-10 doses of Iron Folic Acid (IFA) Syrup every month
- 1.9 crore children of age group 5-9 years were provided 4-5 IFA Pink tablets every month
- 3 crore adolescent of age group 10-19 years provided 4-5 IFA Blue tablets every month
- 1.3 crore pregnant women and 0.6 crore lactating women were provided 180 IFA Red tablets.
l) Rashtriya Bal Swasthya Karyakram (RBSK): During FY 2021-22, due to COVID- 19 pandemic, community level screening activities by Mobile Health Teams of RBSK got affected. As reported by States/UTs in HMIS during April-November, 2021, 4.2 crores children have been screened by Mobile Health Teams. 1.11 crores newborn have been screened at Delivery points under RBSK Program during April-November, 2021.
m) Social Awareness and Actions to Neutralize Pneumonia Successfully (SAANS): SAANS Campaign has been rolled-out in the States/ UTs from 12th November, 2021 – 28th February 2022 with the aim to accelerate the action against Childhood Pneumonia by generating awareness around protect, prevent and treatment aspects of Childhood Pneumonia and to enhance early identification and care seeking behaviours among parents and caregivers. Additionally, awareness generation, promotion and administration of Pneumococcal Vaccine (PCV) has also been included under SAANS campaign for the year 2021.
n) India COVID-19 Emergency Response and Health Systems Preparedness Package (Phase II): Under India COVID-19 Emergency Response and Health Systems Preparedness Package (Phase II), the focus has been given on strengthening of Paediatric Care Facilities at Medical College, District Hospital and Sub-District level facilities. As part of ECRP-II, support has been provided for Paediatric ICU beds, Paediatric HDU beds and Paediatric Oxygen supported beds under dedicated COVID Care Unit at District level. Also, augmentation of Paediatric ICU beds at various levels of facilities has been supported.
o) “Guidelines on Operationalization of COVID-19 Care Services for Children & Adolescents” was released on 14th June 2021 and “Guidelines for Management of COVID 19 in Children (below 18 years)” on 18th June 2021. The Guidelines focus on all aspects of Paediatric Care which includes additional bed capacity for paediatric care during the peak daily cases considering projections for paediatric cases and admissions at different level of facilities; Augmentation of facilities – requirement of drugs, equipment, consumables, Human Resources, capacity building etc; Designating specific areas in the COVID facilities for paediatric care and accompanying parents’/ family members of child; Facility and community level planning; Transport linkages; Management of CoVID in the community settings; IEC Plan; Governance mechanism etc.
3.4 Family Planning
a) Key highlights of NFHS-5 (2019-21):
● India has achieved a replacement level of Total Fertility Rate (TFR). 31 States/UTs have achieved replacement level of TFR.
● Total unmet need has reduced substantially to 9.4% (NFHS-5) from 12.9% (NFHS-4)
● Use of modern contraceptives has increased substantially.
● IUCD use has shown an increase for the first time since NFHS-1. It has increased by 0.6% points, from 1.5 % in NFHS-4 to 2.1 % in NFHS-5.
● 29 States have >70% eligible couples in need of contraception (as against 12 States in NFHS 4). It shows that Family Planning demand generation activities have shown a positive result.
● Overall positive shift towards spacing methods (increase in all spacing methods).
b) The performance of family planning services in FY 2021-22 (upto November 21) is as follows:
● Total Sterilization: 12.51 lakh
● Post-partum IUCD (PPIUCD): 19.08 lakh
● PPIUCD acceptance rate (%) in public health facilities: 23.1 %.
● Contraceptive Injectable MPA (Antara Program): 14.90 lakh doses have been administered
● Non-hormonal Pill Centchroman (Chhaya): 49.44 lakh strips of Centchroman(Chhaya)
c) Mission Parivar Vikas (MPV) – MPV was launched in November, 2016 to substantially increase access to contraceptives and family planning services in 146 High Fertility Districts in seven high focus States with a Total Fertility Rate (TFR) of 3 and above. These Districts are from the States of Uttar Pradesh (57), Bihar (37), Rajasthan (14), Madhya Pradesh (25), Chhattisgarh (2), Jharkhand (9) and Assam (2).
MPV has been expanded to the remaining Districts of seven high focus States and six North Eastern States (Arunachal Pradesh, Manipur, Meghalaya, Tripura, Nagaland and Mizoram) in October, 2021.
MPV Districts have shown substantial increase in improving access to contraceptives.
The performance family planning services of in MPV Districts (146) in FY 20210-22 (Upto November 21) is as follows:
● Total number of Sterilizations: 2.35 lakh sterilization
● Post-partum IUCD (PPIUCD): 5.94 lakh
● PPIUCD acceptance rate (%) in public health facilities : 20.5 %
● Contraceptive Injectable MPA (Antara Program): 6.39 lakh doses
● Non-hormonal Pill Centchroman (Chhaya): 18.2 lakh strips of Centchroman (Chhaya)
3.5 Rashtriya Kishor Swasthya Karyakram (RKSK)
a) Adolescent Friendly Health Clinics (AFHCs): 41.38 lakh adolescents received counselling and clinical services at Adolescent Friendly Health Clinics (AFHCs).
b) Weekly Iron Folic Acid Supplementation (WIFS): 3 crore adolescents had been provided Weekly Iron Folic Acid Supplementation (WIFS) every month besides Nutrition Health Education till November 2021.
c) Peer Educator program: Significant progress has been made in implementation of Peer Educator program with selection of 1.69 lakhs Peer Educators in FY 2021-22 (upto September 2021) to cover for those who have left, grown up or selected fresh in the newer selected districts
d) Adolescent Health Days (AHDs): 64,577 Adolescent Health Days (AHDs), quarterly community & school level activities were conducted till September 2021 to create awareness about adolescent health issues.
e) Ayushman Bharat School Health and Wellness:
● School Health & Wellness Programme (launched in February 2020) is being implemented in government and government aided schools in Districts (including most of the Aspirational Districts) of the country in the first phase of the implementation.
● Two teachers, preferably one male and one female, in every school, designated as “Health and Wellness Ambassadors” (HWAs) shall be trained to transact health promotion and disease prevention information on 11 thematic areas in the form of interesting joyful activities for one hour every week.
● The States have initiated the Health and Wellness Ambassadors training.
● Till 30th November 2021, 1.29 Lakh HWAs have been trained and about 67,391 principals are oriented under the Programme. HWA sessions are gradually starting in the States with reopening of schools.
f) NFHS-5 (2019-21) key highlights:
● 32 States/UTs have shown reduction in early marriages and 25 have shown reduction in prevalence of teenage pregnancies as compared to NFHS-4.
● NFHS-5 (2019-21) has reflected that women aged 15-24 yrs who use hygienic methods of protection during their menstrual period have increased to 77.3% from 57.6% (NFHS-4). 35 out of 36 States/ UTs have shown significant improvement in use of hygienic methods during menstruation.
3.6 Pre-Conception and Pre-Natal Diagnostic Techniques (PC & PNDT):
● As per Quarterly Progress Report (QPR) of June 2021, submitted by the States/UTs, total 72,965 Diagnostic facilities have been registered under the PC& PNDT Act. So far, a total of 2589 machines have been sealed and seized for the violations of the law. A total of 3,201 court cases have been filed by the District Appropriate Authorities under the Act and 617 convictions have so far been secured, leading to suspension / cancellation of medical licenses of 145 doctors.
● NFHS-5 (2019-21) has also recorded improvement of 10 points in the sex ratio at birth at the national level from 919 in NFHS-4 to 929. 23 States/UTs have shown improvement whereas 13 States/UTs show decline in sex ratio at birth.
● Review meetings were conducted in all 36 States/UTs and implementation of PC&PNDT Act was reviewed in all aspects.
● Capacity building of District Appropriate Authorities and PNDT Nodal Officers was conducted in the State of Bihar, Telangana and Andhra Pradesh.
● Training of public prosecutors was organized in Chhattisgarh and Telangana.
4.NATIONAL TUBERCULOSIS ELIMINATION PROGRAMME
Despite a severe second wave of the COVID-19 pandemic this year, the NTEP continued with integrated TB-COVID bi-directional screening, diagnostic and treatment capacity upgrades, and co-located testing for TB (among COVID-19 patient as well as ILI/SARI patients) and testing for COVID-19 (among notified TB patients) apart from periodically updated advisories, directives, and guidance documents being issued to the states.
Large scale active TB case finding campaigns were undertaken with massive screening and testing in communities, health outreach workers and community volunteers were engaged to facilitate surveillance of symptoms within households, doorstep collection of samples and delivery of monthly medicine stock to help patients stick to treatment regimens, and teleconsultations with patients. Private sector TB care facilities were reopened, call centres were fully activated, digital tools were rolled out along with support like direct cash transfers and supplementary food provisions were delivered to people’s homes. Contact tracing systems and testing for TB linked to COVID-19 contact tracing were also quickly set up throughout the country.
During the year 2021, a total of 15,79,410 (Jan-Sep) patients were notified with 95% of patients put on treatment. Treatment Success Rate of notified TB patients was 81% despite the pandemic. Molecular diagnostic capacities were swiftly expanded and additional machines for rapid molecular testing for TB and Drug Resistant TB (DR-TB) deployed. A total of 3,164 CBNAAT/ TrueNat machines are now available under NTEP with at least one rapid molecular diagnostic facility available in each district.
Approximately 17.00 lakhs molecular tests were performed between Jan- June 2021 with an additional 19,3130 first line LPA, 32,600 second line LPA and 1,70,203 liquid culture tests performed.
Due to easy availability of molecular diagnostics, 88,446 children were diagnosed with TB disease this year. The proportion of TB patients with known HIV status has increased and 1.05 lakh PLHIV have been tested using NAAT with 94% of notified TB patients having been screened for HIV.
In addition to the 87 Culture & DST laboratories certified by NTEP, 18 new laboratories are being developed with 28 laboratories being upgraded with LPA facilities. Liquid culture-based DST has been expanded to Linezolid and Pyrazinamide across India and DST capacities are being built for Bedaquiline, Delamanid, and Clofazimine at NRLs. Five Whole Genome Sequencing Platform and one Pyrosequencer has been deployed at national and state level TB laboratories to be initially used for sentinel surveillance of drug resistance. Automated solutions using Artificial Intelligence for LPA result interpretation through Machine Learning (ML) are also being developed.
793 DR-TB centers were made functional to offer decentralized Drug Resistant TB (DR-TB) treatment services which include 173 Nodal DR-TB centers. The access of injection free longer oral MDR-TB regimen with new drugs was expanded in all States/UTs in India. During the year, (Jan -Sep) 37,005 MDR/RR-TB patients were diagnosed and 33,224 (90%) were put on appropriate treatment. This included 10,105 M/XDR-TB patients put on longer oral MDR-TB regimen between January and September 2021.
Under the TB-Diabetes collaborative services, 86% of the TDCs are now co-located with diabetes screening facilities. 82% of all notified TB patients have been screened for diabetes in 2021 (Jan – Sep).
The NTEP has also initiated Sub-National Certification of Districts/States/UTs for achieving “Progress towards TB Free Status” under Bronze, Silver and Gold categories measured with graded milestones of decline in TB incidence compared to 2015 levels. Districts/States/UTs are certified under the categories upon independent verification by a national team composed of National Institute of Epidemiology, WHO India and Indian Association of Preventive and Social Medicine. A total of 3 States/ UTs and another 67 districts across the country laid claims under various categories. The UT of Lakshadweep and the district of Budgam in Jammu & Kashmir has been declared as the First TB Free UT and District in the country.
Under the Nikshay Poshan Yojana (NPY) the NTEP has cumulatively disbursed ~Rs. 1,373 Crores to over 52.53 lakh TB patients as financial support for nutrition needs.
Till July 2021, about 616 districts have moved to DSC based approvals in PFMS. Work is ongoing on integrating Nikshay with SOCH (Strengthening Overall Care for HIV beneficiaries) and with the Ayushman Bharat HWC-CPHC portals.
This year, TB Preventive Treatment (TPT) was expanded to include children above five years, adolescents and adult household contacts of index TB patients with preventive chemoprophylaxis given to 47,695 child contacts of TB patients between Jan and Sep 2021.This will be systematically expanded to cover all States by 2022.
To expand community-based services for prevention and management of TB and to bring services closer to the community, TB has now been integrated with Ayushman Bharat- Health & Wellness Centres. The NTEP completed training of about 3,326 CHOs to provide TB services through HWCs. 829 CHOs were trained on Active Case Finding. Patient Provider Support Agencies (PPSAs) through domestic budgetary resources were approved for a total of 447 districts in 24 States. Through the available modalities, 484 NGOs and Private Provider engagements were made countrywide.
To address the social determinants of health beyond medical interventions, the NTEP has successfully engaged with other Ministries, departments, PSUs, corporates, industries, professional associations, medical colleges and institutions, private healthcare providers, development partners and the community in critical areas of the TB care cascade like treatment, diagnostics, logistics and supply chains, Surveillance and Monitoring, technology-driven interventions, operational research etc. NTEP has managed to reach out to over 400 organizations with 130 of them signing the Corporate TB Pledge (CTP).
This year, despite the raging pandemic, NTEP launched the TB Mukt Bharat Abhiyaan (TMBA) as a Jan Andolan or People’s Movement for TB to further build awareness about TB, address the deep-seated stigma around the disease in the community, raise awareness about the available TB services under the Programme, and generate demand for TB services in the community. The TMBA campaign aims to involve all 134 crore people of the country from all walks of life, including elected representatives and state government leaderships, to create a mass movement and drive community ownership of the government’s efforts.
5. National Programme for Tobacco Control and Drug Addiction Treatment [NPTCDAT]
- Continuous steps are being taken to increase awareness on ill-effects of tobacco use. In this regard, online competitions through MyGov platform are being encouraged for young children and citizens of the country. Under the National Tobacco Control Programme, digitization is being encouraged and an online portal/Management of Informatics System has been developed for the States to provide online reporting of the activities down from the district level. States too are appreciating the importance of the Online reporting / real time data and are participating in this wholeheartedly.
- To gauge the prevalence of tobacco use among youth aged 13-15 years (among grades of 8th, 9th and 10th) and tracking of key tobacco control indicators, the Ministry undertook the Global Youth Tobacco Survey, 2019 (GYTS-4). The National Fact Sheet of Global Youth Tobacco Survey (GYTS-4), 2019 was released by the Union Minister of Health & Family Welfare. As per the results, there has been a decline in the current use by 42% (from 2009 to 2019). This is the first GYTS that also gives us State level estimates apart from the national level estimates.
- India has been committed towards tobacco control. Taking forward the agenda and pursuing the international commitments, India participated in the deliberations held for the ninth session of Conference of Parties (COP 9) of the World Health Organization –Framework Convention for Tobacco Control (WHO FCTC) at Geneva. India also assumed the Presidency of the Meeting of Parties Bureau, to support Parties (countries) to implement the Protocol to Eliminate Illicit Trade in Tobacco Products.
- Substance Use Disorders (SUDs) includes a spectrum of problems caused by the persistent misuse of mind altering substances, and can range from a harmful use to dependence. Considering the importance that physicians are able to effectively identify, diagnose and manage the problems of substance use disorder, the “Standard Treatment Guidelines for the Management of Substance Use Disorders and Behavioural Addictions” was released. These Guidelines have been developed as a resource material for the general physicians in primary care setting to provide them necessary know-how for assessment and management of these disorders.
6. Pradhan Mantri Swasthya Suraksha Yojana (PMSSY)
The Pradhan Mantri Swasthya Suraksha Yojana (PMSSY) envisages creation of tertiary healthcare capacity in medical education, research and clinical care, in the underserved areas of the country. It aims at correcting regional imbalances in the availability of affordable/reliable tertiary healthcare services and also augmenting facilities for quality medical education in the country. The scheme has two broad components:
- Setting up of All India Institute of Medical Sciences (AIIMS);
- Up-gradation of existing Government Medical Colleges/Institutions (GMCIs).
So far, establishment of 22 new AIIMS and 75 up-gradation Projects of existing Government Medical Colleges/Institutions (GMCIs) have been approved under this scheme.
6.1 Six AIIMS under Phase-I:
Six AIIMS approved under Phase- I (AIIMS-Bhopal, AIIMS-Bhubaneswar, AIIMS-Jodhpur, AIIMS-Patna, AIIMS-Raipur and AIIMS- Rishikesh) are already fully functional. All key hospital facilities and services such as Emergency, Trauma, Blood Bank, ICU, Diagnostic and Pathology are functioning.
More than 450 hospital beds increased during this year.
100 PG seats and 150 MBBS seats have been increased during this year.
Dedicated hospitals block for treatment of CoVID-19 patients and CoVID test Lab made functional in these AIIMS during this year.Around 13 AIIMS were providing COVID Treatment and Testing facility with around 2006 beds including 918 Ventilator Beds in ICU were operational during peak of COVID pandemic in June, 2021
6.2 Other New AIIMS under Phase-II, IV, V, VI & VII:
16 AIIMS have been sanctioned/approved by the Cabinet in subsequent phases. Following facilities and services have been made functional in these institutes:
Limited OPD services are already functional in 7 AIIMS viz. Nagpur, Rae Bareli, Mangalagiri, Gorakhpur, Bathinda, Bibinagar and Kalyani. Limited OPD facilities at Deoghar and Bilaspur have started during the year. Limited IPD Facilities for treatment of CoVID -19 patients started at AIIMS Mangalagiri, AIIMS Nagpur and AIIMS Bathinda during this year. CoVID test Lab is also functional in AIIMS Mangalagiri, AIIMS Nagpur and AIIMS Bathinda. 300 bedded IPD has started at AIIMS Gorakhpur with effect from 7-12-2021.
Undergraduate MBBS course with 100 seats per annum per AIIMS is already functional at eight new AIIMS viz. Mangalagiri, Nagpur, Rae Bareli, Kalyani, Gorakhpur, Bathinda, Deoghar, Bibinagar and 50 seats each at AIIMS Guwahati, Jammu, Rajkot and Bilaspur.
Construction is in advanced stages in 8 AIIMS, viz. AIIMS Raebareli, Nagpur, Mangalagiri, Kalyani, Gorakhpur, Bathinda, Bilaspur, and Deoghar. Also, construction work is in progress for AIIMS at Guwahati (Assam), Awantipura (Kashmir), Sambha (Jammu) and Rajkot (Gujarat).
6.3.Up-gradation of existing GMCIs :
The Up-gradation programme broadly envisages improving tertiary health infrastructure through construction of Super Speciality Blocks / Trauma Care Centres etc. and/or procurement of medical equipment at/for existing Government Medical Colleges / Institution.
Since inception of the Scheme, 53 upgradation projects of existing Government Medical Colleges / Institutions have been completed, adding more than 12000 Super-specialty beds including 2000 ICU beds. The Super Specialty Blocks /Trauma Centres constructed in these upgradation projects are also being used as COVID Hospital Blocks. Following 4 projects have been completed during 2021-22 (upto November, 2021):
|
S. No. |
Name of the |
Name of the State |
Phase |
Type of facility |
Total Beds |
ICU Beds |
No. of Super Specialties |
|
1 |
Goa Medical College, Panaji |
Goa |
III |
SSH |
527 |
87 |
11 |
|
2 |
Rajiv Gandhi Institute of Medical Sciences, Adilabad |
Telangana |
III |
SSH |
210 |
42 |
8 |
|
3 |
Agartala Govt Medical College, Tripura |
Tripura |
III |
SSH |
169 |
29 |
7 |
|
4 |
Regional Institute of Ophthalmology (RIO), IMS, BHU, Varanasi |
Uttar Pradesh |
V (B) |
OC |
– |
– |
1 |
7. Medical Education
- The historic National Medical Commission Act was passed by the Parliament in August, 2019. Now, the National Medical Commission has been constituted with effect from 25th September, 2020 and the years old MCI has been dissolved and the Indian Medical Council Act, 1956 has been repealed. The principal change in the regulatory mechanism is that the regulator will be primarily ‘selected’ rather than ‘elected’. The National Medical Commission will steer the reforms in medical education. This will include increase in UG & PG seats along with improved access to quality and affordable medical education and maintaining high ethical standards in medical profession. Some of the key areas in which NMC will work include – implementation of National Exit Test (NEXT) for the medical graduates, guidelines for determination of fee for 50% seats in private medical colleges and Deemed Universities, Regulations for Community Health Providers and rating of medical colleges.
- During the last six years, MBBS Seats increased by 72%, from 51,348 seats in 2014 to 88,120 seats in 2021 and the number of PG seats increased by 78% from 2014 (30,185 seats) to 2021 (55,595 seats).
- Further, during the same period, 209 new medical colleges have been established and now the country has 596 (Govt: 313, Pvt: 283) medical colleges.
- Under the Central Sponsored Scheme for establishment of new medical colleges, establishment of 157 medical colleges have been approved in three phases, of which 70 are functional and remaining will be functional in a few years. Of these 157 colleges, 39 are coming up in the Aspirational Districts of the country thereby addressing the issues of inequity in medical education.
- Rationalization of Minimum Standards Requirements (MSR): The MSRs for establishment of medical colleges have been streamlined. This will reduce the cost of establishment of new medical colleges and increase intake capacity.
- Two years post MBBS Diplomas by National Board of Examinations: Keeping in view the importance of Diploma courses to meet the shortfall of postgraduate students and augment healthcare in remote parts of the country, the National Board of Examinations (NBE) has launched diplomas in eight disciplines namely – Anaesthesia, Gynaecology & Obstetrics, Pediatrics, ENT, Ophthalmology, Family Medicine, Tuberculosis & Chest Diseases and Medical Radiodiagnosis.
- District Residency Scheme for Post-Graduation: The MCI has also notified a Scheme known as District Residency Scheme for compulsory three months training of PG medical students at District Hospitals, an essential component of postgraduate medical training curriculum. Under the Scheme, the second/third year PG students of medical colleges would be posted in the district hospitals for a period of three months.
- The constitution of the National Medical Commission has ushered in a landmark reform in the sector of Medical Education. On similar lines, the Government is striving to bring institutional reforms in nursing and dental education sectors by bringing reformative legislations to replace the existing Indian Nursing Council Act, 1947 and Dentists Act, 1948. To address the long standing vacuum of a regulatory body for various professions included in the allied and healthcare sector, a National Commission for Allied and Healthcare Professions Act 2021 has already been enacted. The basic premise and principled change that is happening in all these professional education sectors is that the Regulator is now being ‘selected on merits’, as opposed to an ‘elected’ regulator.
8. Indian Council of Medical Research (ICMR)
Indian Council of Medical Research (ICMR), New Delhi, is the apex body in India for the formulation, coordination and promotion of biomedical research and is one of the oldest medical research bodies in the world now under the Department of Health Research (DHR).
The Council’s research priorities coincide with the National health priorities such as control and management of communicable diseases, fertility control, maternal and child health, control of nutritional disorders, developing alternative strategies for health care delivery, containment within safety limits of environmental and occupational health problems; research on major non-communicable diseases like cancer, cardiovascular diseases, blindness, diabetes and other metabolic and haematological disorders; mental health and drug research (including traditional remedies). All these efforts are undertaken with a view to reduce the total burden of disease and to promote health and well-being of the population.
ICMR has also demonstrated its commitment to the future of medical research through its professional development training and capacity building. This includes training programs, workshops, and short-term research studentships for those preparing for a career in medicine and medical research. It also includes research fellowships and short-term visiting fellowships for up and coming researchers to expand their skills and knowledge early in their career. ICMR also offers Emeritus Scientist positions to enable retired medical scientists and teachers to continue to carry out research on specific topics.
The impact of ICMR spans across the globe with research collaborations spanning every continent. Through ICMR’s Memoranda of Understandings (MoUs), ICMR has partnered with leading universities from around the world to concentrate efforts on leading health issues such as cancer, diabetes, infectious diseases, and vaccine development. These collaborations facilitate the exchange of scientific information, training, joint projects, and co-authorship of meetings, workshops, seminars, and symposia presentations.
Intramural Research
Intramural research is carried out through a countrywide network 27 institutes/centres with multiple field stations, 14 work in the area of communicable diseases; 6 in Non-Communicable Diseases, 1 in diseases related to Reproductive and Child Health (RCH); 1 in nutrition and nutritional deficiencies, 3 in disease related to Basic Medical Sciences including haemoglobinopathies and traditional medicine, 1 in the area of animal breeding and research and 1 is patient care and research centre.
Extramural Research
Extramural research is promoted by ICMR through- Setting up Centres for Advanced Research in different research areas around existing expertise and infrastructure in selected departments of Medical Colleges, Universities and other non-ICMR Research Institutes. Task force studies are also carried out which emphasize a time-bound, goal-oriented approach with clearly defined targets, specific time frames, standardized and uniform methodologies, and often a multi-centric structure.
Open-ended research on the basis of applications for grants-in-aid received from scientists in non-ICMR Research Institutes, Medical colleges, Universities etc. located in different parts of the country.
Achievements during the year:
- COVID-19 Pandemic: ICMR has been at the forefront in the fight against Covid-19. The major achievements in this area are listed below:
- COVID-19 Testing: COVID-19 has emerged as a global pandemic and has caused significant morbidity and mortality all over the world. The testing capacity has been expanded significantly in the country. RT-PCR based testing capacity has been in almost all parts of the country from 1 lab in January 2020 to a total of 3011 labs in October 2021 (1336 Govt. &1677 Private Labs). All labs have been established after due diligence and ascertaining adequate checks and balances to ensure high quality testing. NABL accreditation with specified scope has been ensured for all private laboratories. The lab network conducted more than 22 lakh cumulative tests on May 26, 2021 as compared to only 20,000 tests on March 23, 2021. A total of 60 Crore tests have been conducted till now.
- COVID-19 Vaccine:
Covaxin: Covaxin is an indigenous inactivated whole-virion SARS-CoV-2 vaccine BBV152. ICMR has partnered with Bharat Biotech International Limited (BBIL) to develop a fully indigenous vaccine for COVID-19 using the virus strain isolated at ICMR’s National Institute of Virology (NIV), Pune. The vaccine has been found to be 78% effective in the phase 3 clinical trial results. The neutralizing antibodies elicited by immunization with BBV152/Covaxin were found effective against alpha, kappa, gamma and beta variants. Emergency Use Authorization (EUA) is given and is rolled out for vaccination.
Covishield: Apart from the fully indigenous vaccine development initiative, ICMR collaborated with Serum Institute of India and Oxford University to fast-track phase I/II clinical trials of the live attenuated recombinant vaccine for COVID-19 developed by the Oxford Group. The vaccine has received the Emergency Use Authorization (EUA) and is rolled out for mass vaccination.
- COVID-19 Vaccine Effectiveness: ICMR has undertaken several studies to demonstrate the effectiveness of COVID-19 vaccines against the variants of concern (Alpha, Beta, Gamma, Delta) as well as in real- world settings. ICMR has also demonstrated that COVID-19 vaccine is effective in preventing mortality (Dose 1: 96.6% & Dose 2: 97.5%).
- Drone-based delivery of COVID-19 Vaccine: Union Minister for Health & Family Welfare, Shri Mansukh Mandaviya, launched ICMR’s Drone Response and Outreach in North East (i-Drone).i-Drone is another pioneering initiative undertaken by ICMR to transform India’s health ecosystem. First sortie with vaccines was conducted from Bishnupur District Hospital, to PHC Karang today. Bishnupur is located in the plains and PHC Karang is located on the island of Loktak Lake of the Bishnupur district. It takes approx 2.5 hrs (25 Km by road, 3 Km by boat and further 2 Km by trek) to reach PHC Karang from District Hospital Bishnupur. Whereas the drone took only 15 minutes to reach the PHC Karang from Bishnupur District hospital. This is the first such initiative of vaccine delivery through drone from plain to island in south-east Asia.
- COVID-19 Third &Fourth National Serosurvey: The 3rd&4th round of national sero-survey for #COVID19 demonstrated overall sero-prevalence of 24.1% &67.6% in the entire population respectively. The 4th serosurvey demonstrated that a third of the population did not have antibodies (Still ~ 40 crores vulnerable). Thus, states/districts/areas without antibodies run the risk of infection waves.Implications of 4th Round of National Sero-Survey show that there is a ray of hope but there is no room for complacency. Non-essential travel must be discouraged and travel only if fully vaccinated.
- Other Communicable Diseases:
- End TB by 2025: In efforts towards TB elimination, the National TB Prevalence survey was conducted in 625 clusters in all states/UTs covering around 5 lakh population to assess the true burden of TB. It has helped in identifying the hotspots in the country where accelerated efforts are required.
- Malaria Elimination Research Alliance (MERA) India: ICMR funded 32 projects under MERA India initiative, including eight individual studies, and 24 multi-centric projects under Task Force mode on four themes viz low-density infection detection (LDI); vector bionomics, Geographical Information System and community behavior. The salient features of the MERA-India multi-centric projects include mentoring by the experts; standard common objectives and methodology to maintain research quality and uniformity of data generation; and extensive peer-review. As a step towards capacity-building and in order to provide training to the young investigators, MERA-India organized workshops at ICMR-NIMR in the multi-centric project themes. On the occasion of World Malaria Day, MERA-India organized a virtual international symposium in April 2021. As part of MERA-India’s research outreach activities, MERA-India is carrying out two virtual lecture series-“Lecture Series on Infectious Diseases” and “Distinguished Lecture Series”, in which each month renowned scientists and experts from different fields are invited to deliver lectures. These lectures have witnessed huge participation with attendees from across the globe
- Released the first landmark report on the “Impact Evaluation of Antiretroviral Treatment, under the National AIDS Control Programme in India”. Antiretroviral Treatment (ART), the multidrug treatment for HIV infection, is provided free to adults and children living with HIV across India by NACO.The study demonstrated the high impact of antiretroviral treatment and showed that the chance of death was halved among people on ART after 5 Years of treatment. The probability of Tuberculosis was lower among persons on ART as compared to those not on ART. Cohorts of people who had initiated ART in 2012 and 2016 and continued taking treatment underwent viral load testing and over 90% showed that the virus in their blood was adequately suppressed. Over 70% of beneficiaries of ART reported ‘good’ or ‘very good’ quality of life overall and 82% were productively employed. The ART programme under NACP was found to be very cost-effective.
- Non-Communicable Diseases & Nutrition
- Released “Clinico-pathological Profile of Cancers in India: A Report of Hospital Based Cancer Registries, 2021”: It is based on eight-year data of cancer cases from 96 HBCRs’ under the NCRP. The data pertains to all diagnosed and treated patients of confirmed malignancies reported to these centres across the country. The report presents a general overview of the proportion of cancer sites relative to all sites, cancers in sites associated with tobacco use, childhood cancers and detailed chapters for cancers in various organs sites, which include head and neck, gastrointestinal tract, lung, prostate, central nervous system, thyroid, kidney, bladder, childhood and gynaecological cancers including breast. A total of 1332207 cancer cases were registered from 96 hospitals under the NCRP during 2012-19.Out of 610084 cancers, 319098 (52.4%) cancers were reported in males, and 290986 (47.6%) in females. Childhood cancers (0-14 years) comprised 4.0% of all cancers.Cancers in sites associated with tobacco use comprised 48.7% of cancers among males and 16.5% among females.
- Released a report titled “Profile of cancer and related health indicators in the Northeast Region of India”.The report projects that the number of new cancer cases in the northeast region (NER) is likely to increase to 57,131 by 2025, in comparison to the estimated 50,317 in 2020. These estimates are based on cancer data compiled by eleven Population Based Cancer Registries (PBCRs) in all the eight states. The Report also includes data from seven Hospital Based Cancer Registries (HBCRs) in Assam, Manipur, Mizoram and Tripura from 2012 to 2016.
- Released MUDRA Toolbox a collective effort by seven leading centres in IndiaNIMHANS (Bangalore), AIIMS (New-Delhi), SCTIMST (Trivandrum), NIMS (Hyderabad), Apollo Hospital (Kolkata), Manipal Hospital (Bangalore), and Jawaharlal Nehru Medical College. MUDRA Toolbox is a pioneering initiative undertaken by ICMR Neuro Cognitive Tool Box (ICMR-NCTB) consortium to transform India’s dementia and mild cognitive impairment research and clinical practices.
- Released First comprehensive estimates of disease burden from neurological disorders and their trends in every state of India from 1990 to 2019
- India Hypertension Control Initiative: The project has been expanded to 100 districts in 19 States covering more than 7800 health facilities. More than 1.7 million hypertensive patients and more than 0.4 million diabetes patients have been registered. Blood pressure control rates in patients during quarter one of 2021 ranges from 33% to 61 % among the States.
- Stroke Care pathways using Mobile Stroke Unit: Stroke Units have been set up at AMC, Dibrugarh, TMC and BCH, Tezpur. Mobile stroke units are in place at Tezpur and Dibrugarh. TMC, Tezpur has no neurologist and physicians have been trained to manage stroke. TMC, Tezpur stroke unit has done thrombolysis in 4 patients whereas BCH has done thrombolysis in 6 patients so far. The MSU at Tezpur completed its dry run. Two patients called for MSU at Tezpur. One was a hemorrhagic stroke case, whereas the other was that of stroke mimic. The CT scanner in MSU at AMC, Dibrugarh was used in one patient to identify ischemic stroke patients and was a thrombolytic drug within 3 hours of symptom onset.
- Mission Delhi: This project uses motorcycle ambulances to provide pre-hospital thrombolysis to STEMI patients. The call centre at AIIMS received 263 emergency calls for which a motorcycle ambulance was sent and 586 ECGs were done and transmitted to AIIMS. Of these 114 were cardiac emergencies with ECG changes, there were 36 acute coronary syndrome cases of which 18 were STEMI cases. The team gave thrombolytic therapy to 11 of these STEMI patients at their door and the seven patients who were not eligible for thrombolysis were brought to hospital and Primary PCI was done in 3 cases and rescue PCI in 2 cases.
- STEMI ACT: This project aims to improve thrombolysis rates using hub and spoke model in a district. The hub is a medical college and spokes are CHCs, Civil hospital and District hospital. This project has been initiated in 7 districts of 6 States. The centre at Shimla, HP has successfully implemented the project in Shimla district where the spokes thrombolysed 52 STEMI patients and hub thrombolysed 60 patients. In April 2021, there were 47 cases of ACS of which 27 were STEMI cases. Of these 27 patients, 16 were referred to hub hospital by spokes; 10 out of 16 patients were thrombolysed at SPOKE centres ( civil Hospital, Rohru; MGMS, Khaneri, Civil Hospital, Nerva; CHC, Kotkhai); 5 out of 11 patients were thrombolysed at Hub hospital. Twelve patients were out of window period.
- Biomedical Informatics (BMI)
- National COVID-19 Testing Data Management System
The system collects individual demography, travel history and category along with test information including sample type, genes and CT values for all the testing types (RT PCR, CB NAAT, TrueNAT and RAT). The system gets input from (i) Laboratories submitting data directly to the system (ii) Sample collection data through linkage with RTPCR app (iii) Data pushed by API from state (UP, Bihar, Andhra Pradesh, Kerala and Telangana) applications. The system has collected approx. 60 crore fifty eight lakh eighty five thousand seven hundred sixty nine (as of Oct28, 2021) individual testing records from multiple labs across all the states of India. Different stakeholders (Cabinet Secretariat, PMO, MoHFW, ICMR, State Health Secretaries, State Surveillance Officers, District Magistrates/Collectors and District Surveillance Officers) have been provided with role based dashboards providing real-time access to tests conducted, positives, test positivity rate, State and Lab TATs. In addition to dashboards, the stakeholders (NDMA, MoHFW, NIC, NHA and States) have also been provided with specific APIs for feeding data into their applications.
- National COVID Kit Validation System
The system is developed to improve efficiency in the kit validation process. The system consists of three modules: (i) Vendor module where a vendor registers the kit for validation, view progress of the kit validation process and visualize/download validation results (ii) ICMR module where ICMR can view/accept/reject the submitted kit information, assign the kit to validation centre and approve the results for dissemination (iii) Validation centre module where the selected centre uploads the validation results after testing the kit. Real-time data analytics have been developed for different stakeholder and regular reports are being shared with stakeholders
- Lab Capacity and QC/QA Management System
COVID QA-QC portal has been designed and developed for maintenance of quality control data for labs. All the labs registered for COVID testing in the ICMR portal, undergo a quarterly quality control protocol where few positive and negative samples are sent to the QC lab for testing. QC process is a three-tier process. On the top level is NIV, Pune which is the national QC lab. All the QC labs perform the quality control activity with NIV Pune. The testing lab undergoes the same process with their designated QC labs. Presently, the portal has been launched for all the RT PCR labs in the country. The testing lab fills in the sample details in the portal. Similar sample details are entered by the QC labs. Both the test results are visible to ICMR, which are then marked as concordant or dis-concordant. The report is then visible online to both the QC and testing labs, thus enabling transparency in the entire process.
- Jal Jeevan Mission Portal
The department has developed an online portal for India’s Jal Jeevan Mission for collecting data on water quality that will make it possible for users to get quality of drinking water tested through a network of nearly 2,000 labs across the country as well as the ones submitted individually using Field Test Kit (FTK).
- New Infrastructure
- Established Regional Medical Research Centre, Gorakhpur to address the regional health challenges of eastern belt of Uttar Pradesh. It was inaugurated by Hon’ble Prime Minister Shri Narendra Modi.
- Laid the foundation stone of new building of ICMR School of Public Health at National Institute of Immunology, Chennai by Hon’ble Union Health & Family Welfare Minister, Dr Mansukh Mandavia.
- Inaugurated a state of art BSL-3 facility at ICMR-National JALMA Institute of Leprosy and other Mycobacterial Diseases, Agra for undertaking research on high-risk pathogen. It was inaugurated by then Hon’ble Union Health & Family Welfare Minister, Dr Harsh Vardhan.
- Inaugurated new eco- friendly building of ICMR- National Institute for Research in Environment Health, Bhopal. It was inaugurated by the then Hon’ble Union Health & Family Welfare Minister, Dr Harsh Vardhan.
- Other Achievements
- Established “ICMR at IITs’ ‘ by setting-up Centres of Excellence (CoE) for strategic Make-in-India product development and their commercialization in the medical device and diagnostics sector.
- Released the ‘National Guidelines for Data Quality in Surveys’. The guidelines for Data Quality in Surveys’ aims to provide comprehensive guiding principles and best practices for mitigating errors and biases that may occur during survey design, data collection and analysis, thereby ensuring data quality in surveys, specifically for demographic, health and nutrition surveys.
- UNEP and ICMR launched a new collaborative project- “Priorities for the Environmental Dimension of Antimicrobial Resistance (AMR) in India”, marking an important step towards recognizing and addressing the environmental dimension of AMR.
- Under various Fellowship Programs and financial support Schemes ongoing in the ICMR-Human Resource Development (HRD) Division, ICMR selected a total of 138 candidates (126 for Life Sciences and 12 candidates for Social Sciences) for Junior Research Fellowship (JRF)-2020, through national level exam, the result of 2021 is still to be declared. A total of 1252 medical/dental undergraduates were selected for ICMR-Short Term Studentship (STS)-2020 Program. Results for ICMR-Nurturing Clinical Scientists (NCS) Scheme are under review and still to be declared. Eleven (11) candidates were selected for the Award of ICMR-Post Doctoral Fellowship (PDF) in the year 2021. The MD/Ph.D. Program is ongoing in three universities and only six (06) students have joined in the year 2021. Financial support for pursuing MD/MS/MCh/DNB/DrNB/MDS thesis was also granted to a total of 102 fellows. A total of four Adjunct Scientists have joined in the year 2021. There are two ongoing Chairs for ICMR-Dr. C.G. Pandit National Chair and in ICMR-Dr. A.S. Paintal Distinguished Scientist Chair, three are ongoing and two Chair positions are vacant for which applications are under review.
- Under International Cooperation, the existing partnerships in Health Research with various international organizations/agencies continued with 3 new MoUs with NHRC, Nepal; FIND, Switzerland GARDP Foundation, Switzerland & 1 LoI with Min. of Health & Social Protection and Min. of Science, Technology & Innovation of Colombia signed during the year.
- One working level virtual meeting with FORTE, Sweden and a interactive meeting with NHRC, Nepal to discuss future plans were held. A total of 157 international projects (between Jan,21 to Oct, 2021) were approved in five meetings of the Health Ministry’s Screening Committee (HMSC). Also organized the visits to ICMR Hqrs. for delegations from Myanmar, Brazil, Germany and Colombia.
- ICMR has processed 165 fellowships (approx.) and 30 ad-hoc projects (approx.) to support health research in different medical colleges, institutes & universities.

Hon’ble PM inaugurated the new building of ICMR-RMRC, Gorakhpur on 7th December, 2021. Having a look of the Model of new building with UP Chief Minister Shri Yogi Adityanath and Governor Ms Anandiben Patel and DG ICMR


Hon’ble HFM launching the I-drone initiative of ICMR for vaccine delivery in remote hard to reach areas on 4th October, 2021
Central Government Hospitals
8.1Atal Bihari Vajpayee Institute of Medical Sciences and Dr. Ram Manohar Lohia Hospital (ABVIMS & Dr RML Hospital)
- Starting of MBBS Course: Directorate General of Health Services/ Ministry mandated PGIMER and Dr. RML Hospital to start MBBS course with intake of 100 students from academic session 2019-20. The name of the Institute has also been changed to “Atal Bihari Vajpayee Institute of Medical Sciences and Dr. RML Hospital”. Now the Institute has state of the art Labs, Dissection Hall, Examination Hall, Lecture Theatres, Museum etc.
- Super Specialty Block: Hospital has planned to construct a new 600+ bedded Super Specialty Block (SSB) comprising 3 basements + GF+ 11 Upper Floors at a vacant plot available at G-Point of the Hospital. The EFC in its meeting held on 18.02.2019 approved the project at a total cost of Rs 572.61 Crore. CPWD has been nominated as the Project Management Consultant. Tenders have been awarded by CPWD and the expected period of completion of the project is 24 months.
- New Hostel Block: The Institute has planned to construct 824 Rooms New Hostel Block at the vacant land available in the campus. The total cost of the project is 178 crore. HSCC is the Project Management Consultant. The work up to the 7th Floor level has been completed.
- Dr. RMLH has already received Paediatric Cath Lab and soon the department of Paediatric Cardiology will be started and it will be first of its kind in the country in a Government hospital.
- Dr. RMLH is in the process of procurement of a Robotic System. This will be used by different surgical specialities to perform complex operations. This gives enormous benefit to patients, who have to undergo difficult and complicated surgery.
- Doctors of Dr. RMLH have already been trained for Liver Transplantation and this will be started in near future after getting all necessary approvals.
- E-office has been started in Dr.RMLH
- Dr. RMLH was made the first Corona nodal centre in the country by MoH&FW, GoI. Corona patient management has been done in OPD and also dedicated admission facilities and ICU facilities were created. RtPCR tests for all suspected patients were routinely done. In addition to the above, 150 bedded COVID Care Centre at Vishwa Yuva Kendra, Chanakyapuri was managed by Dr.RMLH.
8.2 Lady Hardinge Medical College & Associated Hospitals.
1. LHMC with Associated Hospital actively participated for providing treatment facilities in COVID-19 PANDEMIC which started in 2020.
Following facilities were created: –
- Red Zone
I. Ward – 24 + 22 = 46
II. COVID ICU Beds = 30
III. Orange Zone Beds = 103 (for suspected cases)
- Various infrastructures added for Treatment of COVID-Patients.
I. Capacity of Ventilatory beds increased by 30 beds.
II. Number of BIPAP machines – 32.
III. HFNO-facilities added.
IV. Sufficient quantities of Pulse Oxymeters available, PPE Kits, N-95 masks and others consumables.
V. Number of beds increased with O2 supply >50 beds.
VI. Flu-Clinic for screening of COVID patients.
- COVID-19 TESTING FACILITY:
I. LHMC was one of the First institution to start COVID-19 testing facility in shortest possible time for testing by following methods:
- RTPCR
- CB NAAT
- TRUNAT
Initially services were provided to all major hospitals where testing facilities were not available. More than 40,000 cases have been tested.
II. State of Art- Sampling Centers constructed for taking samples.
- The LHMC team of doctors and paramedical staff ran YMCA COVID-CARE CENTRE.
- LHMC Doctors were part of the Central team for Inspection of facilities and training in various states.
- LHMC provided facilities for both COVID and NON-COVID patients in all departments including maternity and child care even during lockdown and its aftermath.
- Creative Problem Solving Initiatives:
I. Telemedicine facilities
II. Blended teaching
III. Students focused youth wellness initiatives, including self-help groups by providing counseling facilities.
2. Comprehensive Redevelopment Plan (CRP) of LHMC:-
(a) ONCOLOGY BLOCK and ACADEMIC BLOCK are ready for possession and likely to be handed over to LHMC by HSCC before 31st December 2020.
(b) Accident Emergency and OPD Blocks are likely to be ready by 31st March 2021.
3. Post-Graduate Seats: 24 Post-graduate have been increased in LHMC against the EWS quota.
4. Teaching Activities: For Undergraduates, Postgraduates, Post-doctoral courses.
(a) In COVID-Situation, a combination of On-line Teaching along with practical training were being carried on keeping COVID-19 protocols under consideration.
(b) Regular clinical meetings were held ONLINE on Microsoft Teams.
(c) Post-graduate examination was held through Video-conferencing testing practical skills and theoretical knowledge.
5. Annual convocation was held on 12-12-2020 through Video-Conferencing with Hon’ble HFM as Chief Guest.
6. Lab Information System (LIS) as a part of Hospital Management Information System (HMIS) was initiated in LHMC to provide Computer generated lab reports and can be seen by treating doctors for taking quick decisions on treatment of patients.
7. LHMC & Associated Hospitals are in the category of Super-Speciality hospitals for PMJAY.
8.3 Safdarjung Hospital
1. COVID – 19 Pandemic Management: –
The Safdarjung Hospital has been actively involved in the management of COVID – 19 patients as per the guidelines of ICMR and the instructions of Dte.GHS MOHFW i.e hemogram, coagulation profile and biomarkers in Covid 19 positive patient etc.
a). The whole Super specialty Block (SSB) is converted into a dedicated separate block for treatment of COVID-19 patients.
b) Establishment of 44 bedded makeshift hospitals, including 28 ICU Beds.
c) Enhancement of oxygen capacity by establishing PSA Oxygen plants (Capacity of 0a MQ and LMO capacity (60MT).
d) 27 ICU and 20 Non lcu beds established for dealing with COVID Paediatric patients
e) A dedicated Control Room established in Safdarjung Hospital to function round the clock.
c) A separate hi-tech COVID-19 Lab. to do RTPCR, and facilities in NEB & other departments for Truenet, Covid 19 Rapid Antigen Test, Covid 19 Elisa test started in Safdarjung Hospital.
d) The SARI Ward was started with concurrence of District Magistrate in New Emergency Block, Safdarjung Hospital for separate management of cases of sub-acute respiratory illness cases.
e) A dedicated Core team constituted for COVID-19 management consisting of Doctors from Anesthesia, Medicine, Respiratory deptts etc. Separate section was created in SSB for patients of Gyne&Obs and Pediatrics.
f) A Training programmer was being conducted for JR/SR/Nursing staff & Intern on weekly basis to deal with COVID – 19 management.
g) Awareness programme i.e handwashing steps, social distancing, importance of masks and use of sanitizations in hospital for prevention of COVID – 19 infection were conducted for the patients & their relatives coming to the hospital in addition to hospital staff working in various locations of SJH/VMMC.
k) Separate fever clinic & sample collection center (RTPCR) started for COVID-19 patients in Old Casualty Block, SJH.
i) Uninterrupted patients care services were maintained in most of the departments of Safdarjung Hospital and also regular Dialysis for Non-Covid-19 patients is being conducted regularly.
j) Separate Ambulances were engaged for Transportation of COVID-19 patients and dead bodies.
k) Teams are being constituted for potential vaccinators for COVID -19 vaccination drive.
l) Fire safety drills, training & awareness programmes were continuously conducted for fire management in Safdarjung Hospital.
2. 40 LDC’s and 8 PWD candidates had joined in the last three months.
3. A programme of “AaoSathchale” has been started to provide needful help to the patients and their attendants.
4. Status of Admission/Operations: –
Total number of In-patients (admitted) and operations conducted in this hospital: –
|
Admission Jan – Nov 2021
|
Major Operation Jan – Nov 2021 |
Minor Operation Jan – Nov 2020 |
Total Operation Jan – Nov 2020 |
|
119971 |
27642 |
9385 |
37027 |
5. Statistics (X-Ray Examinations)
|
Year |
No. of X ray Examinations |
|
January to November |
3,90,482 |
6. Statistics Deliveries in Department of Obs&Gynae :-
|
Year |
Number of deliveries |
|
January to Nov 2021 |
15,697 |
7. OPD Attendance :-
|
Year |
Number of OPD Patients |
|
January to Nov 2021 |
18,25,878 |
8. Sports Injury Centre: – Patients attendance/surgeries
|
S.NO. |
Year |
SIC OPD |
Physiotherapy OPD |
IPD(SIC) |
O.T |
|
1 |
Jan to Oct 2021 |
57,395 |
37,497 |
1900 |
1622 |
8.4 NEIGRIHMS, Shillong
Major Achievements at NEIGRIHMS, Shillong, from 1st January, 2021 till date of the FY 2021-22:
A: LAND
The District Collector, East Khasi Hills Revenue , has formally handed over the 20 Acres of Additional Land to NEIGRIHMS, on the 23rd November 2020, for the construction of the Dwelling Units for Faculty, Group A, B & C categories.
B: Infrastructure Development
Institute set up the COVID-19 ICU of 10 beds, Isolation Wards for 40 beds and Screening Area for COVID patients catering the entire region.
∙ Institute entered into an agreement with Government of Arunachal for having cashless treatment for the people of Arunachal at NEIGRIHMS under the CMAAY Scheme (Health Insurance Scheme).
∙ Institute had imparted ICU training to doctors of the State Government with regard to ICU COVID Care.
∙ Institute has set up the COVID teleconferencing in all OPDs for the benefit of the patients.
∙ Institute has increased the departments for hospital user charges at subsidised rates in order to generate revenue.
∙ Institute has taken over the following buildings of the new projects for conversion into COVID Quarantine Centres.
Guest House of 48 rooms.
Nursing Hostel – 1 of 88 bed capacity
Nursing Hostel – 2 of 110 bed capacity
Institute has also taken over the Under Graduate Hostels I & II for accommodating the new batch of MBBS students.
The new Nursing College Building along with the hostel will be handed over by 31st December 2020.
Status of the Construction as on 16.12.21 is as follows:-
|
Building Name |
Physical Progress (% Completed) |
Financial Progress (In crores)
|
Actual Date of Start of Works |
Tentative Date of Completion
|
|
U.G Medical College |
79% |
|
115 24.03.2017 |
Jan-22 |
|
Regional Cancer Centre & Guest House |
73%
|
|
61 24.03.2017 |
Mar-22 |
|
Nursing College & Hostels |
100%
|
|
63 24.03.2017 |
Completed
|
|
Ancillary Building |
55% |
|
18 24.03.2017 |
Mar-22 |
|
Facility |
Current Status of Completion |
Scheduled Completion
|
Expected Completion
|
|
Nursing College |
100% |
|
23.03.19 Completed. Occupation certificate (OC) received |
|
Nursing Hostel-1 |
100% |
|
23.03.19 Completed. OC received |
|
Nursing Hostel-2 |
100% |
|
23.03.19 Completed. OC received |
|
Nursing Dining |
100% |
|
23.03.19 Completed. OC received |
|
Guest House |
100% |
|
23.03.19 Completed. OC received |
|
U.G Hostel-1 |
100% |
|
23.03.19 Completed. OC received |
|
U.G.Hostel-2 |
100% |
|
23.03.19 Completed. OC received |
|
Internee Hostel |
100% |
|
23.03.19 Completed. OC received |
|
U.G. Medical College |
77%
|
|
23.09.19 31.01.22 |
|
Regional Cancer Centre |
67%
|
|
23.09.19 31.03.22 |
|
U.G Hostel-3 |
80% |
|
23.09.19 31.01.22 |
|
U.G Hostel-4 |
80% |
|
23.09.19 31.01.22 |
|
Ex Development |
62% |
|
23.09.19 31.01.22 |
|
STP,WTP,ETP |
75% |
|
23.09.19 31.01.22 |
8.5 Regional Institute of Medical Sciences, Imphal
Regional Institute of Medical Sciences was set up in 1972 and has been functioning under the Ministry of Health and Family welfare since 1st April, 2007. RIMS is an Institute of regional importance catering to the needs of the North Eastern Region in the field of medical education by providing undergraduate and postgraduate courses. RIMS is a 1,200 bedded teaching Hospital equipped with modern state of the art equipment and teaching facilities. The Hospital provides services to a large number of patients both out-door as well as indoor patients and admit over forty thousand patients in a year. The institute has so far produced 3560 medical graduates and 1988 specialists.
|
Sl. No. |
Name of Course |
Number of seats |
Quotas |
|
1 |
MBBS |
125 seats per annum |
15% All India Quota |
|
2 |
MD/MS/DCP |
148 seats per annum |
50% All India Quota |
|
3 |
M. Ch./D.M. |
05 seats per annum |
100% All India Quota |
|
4 |
M. Phil. |
06 seats per annum |
Open Beneficiary states of RIMS |
|
5 |
B. Sc. Nursing |
50 seats per annum |
All Beneficiary states of RIMS |
|
6 |
BDS |
50 seats per annum |
15% All India Quota |
|
7 |
BASLP |
10 seats per annum |
All Beneficiary states of RIMS |
|
8 |
M.Sc. (Nursing) |
8 seats per annum |
All Beneficiary states of RIMS & 1 seat earmarked for children of RIMS employee |
2. The courses being run along with intake capacity in the institute are as follows:
2.1 Allocation of Seats for undergraduate courses:
The number of annual admission to MBBS courses is 125 students. The detail of these seats is as under:-
|
Sl. No. |
Name of State |
MBBS |
BDS |
B.Sc. Nursing |
|
1 |
All India Quota |
19 |
7 |
– |
|
2 |
Arunachal Pradesh |
7 |
4 |
5 |
|
3 |
Meghalaya |
13 |
7 |
5 |
|
4 |
Mizoram |
7 |
4 |
5 |
|
5 |
Manipur |
30 |
13 |
20* |
|
6 |
Sikkim |
5 |
3 |
5 |
|
7 |
Tripura |
13 |
7 |
5 |
|
8 |
Nagaland |
10 |
5 |
5 |
|
9. |
NE Open- All Beneficiary states of RIMS (except Assam) |
10 |
– |
– |
|
10. |
EWS |
11 |
– |
– |
|
Grand Total |
125 |
50 |
50 |
|
* including 4 seats earmarked for children of RIMS employees.
2.2 Distribution of P.G. seats
50% (73-74) seat distribution of Beneficiary States of RIMS, Imphal
|
Course |
State |
No. of seats |
Total seats |
|
|
Sponsored |
Open |
|||
|
Postgraduate (MD/MS/DCP) |
Arunachal Pradesh |
8 |
2 |
10 |
|
Manipur |
8 |
2 |
10 |
|
|
Meghalaya |
8 |
2 |
10 |
|
|
Mizoram |
7 |
2 |
9 |
|
|
Nagaland |
7 |
2 |
9 |
|
|
Sikkim |
7 |
2 |
9 |
|
|
Tripura |
8 |
2 |
10 |
|
|
RIMS AIQ Graduate |
|
2 |
2 |
|
|
NON RIMS Graduates of beneficiary States (except Assam) |
|
5 |
5 |
|
|
|
|
|
|
74 |
2.3 ACADEMIC ACHIEVEMENT
The objective of this premier institute is to impart quality medical education and has produced a number of medical doctors/specialists and health care providers. On the basis of the record maintained by the institute number of the students passed out so far as on 31.10.2021 is as under:
a) Total no. of MBBS doctors passed out – 3560
b) Total no. of MD/MS/DCP passed out – 1968
c) Total no, of M.Ch. students passed out – 20
d) Total no. of M.Phil. (Clinical psychology) – 67
e) Total no. of B.Sc. (Nursing) Passed out – 282
f) Total no. of B.D.S. passed out – 143
3. MANAGEMENT OF THE INSTITUTE
The Institute and its teaching hospital is under the administrative control of the Director, RIMS, Imphal. The Board of Governors of the Institute is headed by the Union Health Minister as its President.
The Executive Council is chaired by the Secretary, Ministry of Health & Family Welfare, Govt. of India. The other committees have also been constituted such as the Standing Finance Committee, Academic Sub-Committee etc.
The Medical Superintendent is the overall in-charge of the hospital, who looks after the day to day functioning of the hospital. The functioning of the different departments is directly under the respective heads of department. Key areas such as the Casualty, CSSD, Stores, Hospital Waste Management, etc are looked after by designated officers (medical doctors) under the supervision of the Medical Superintendent.
4. STAFF STRENGTH IN RIMS
|
Sanctioned Posts |
Filled |
|
1936 |
1437 |
5. NEWLY PROCURED EQUIPMENTS/INSTRUMENTS
The list of newly procured major equipments for RIMS Imphal for the year 2020-2021(as on 30th Nov., 2021) are as follows:-
- 64 Slice CT Scan Machine.
- 32 Slice CT Scan Machine.
- PSA Plant 200 D-type Cylinder.
- 3 Nos. of Dialysis Machine.
- 60 nos. of Oxygen Concentrator.
- 4 nos. of Modular OT.
- 2 nos. of LMO tank 10000 KL Capacity
- 850 nos. of D-type Cylinder
- 500 KVA DG Set
- -80 Deep freezer
- 1 set of Conventional Radiotherapy Simulator
- 1 set of 8 channel EMG/NCS/EP system
- 2 sets of 64 channel video EEG machines.
6. OTHER ACHIEVEMENTS
- The number of MBBS seats at RIMS, Imphal increased from 100 to 125 per annum. Out of the 25 seats increased, 11, 10 and 4 seats are reserved for Economically Weaker Section (EWS), NE open and All India Quota (AIQ) respectively.
- MD CROUSE IN Sport Medicine has been started from the academic session 2020-2021 with 1 seat annually.
- 51 numbers of beds have been increased in the Radiotherapy Ward.
- Level 1 Trauma Centre at RIMS, Imphal was inaugurated during the year under report.
- 2 Separate OT for Obstetric and Gynaecology Department was commissioned and became functional during the year under report.
- New Dialysis Centre with 10 new Haemodialysis Machines was inaugurated and became functional.
- The institute hospital has witnessed heightened activity during the year under report. The OPD attendance increased to 7.80 lakh patients. 1.44 lakh patients were treated in the Casualty. Number of in-patients admitted was 0.99 lakh. The increased hospital footfall may be because of many diagnostic tests made available free of cost, implementation of CMHT, PMJAY, increased health consciousness & awareness of the populace. Number of tests and investigations have also increased greatly. In the Biochemistry Department 8.65 lakh investigations were done. In the Radiodiagnosis Department nearly 14,000 CT Scans and more than 1 lakh X-Rays were done. Similarly, investigations in the Microbiology and Pathology Department increased significantly.
- Inauguration of New MRI Block with 3-Tesla MRI Machine, New PG Ladies Hostel- 100 Capacity (G+3), New Neuro ICU Block and College of Nursing New Block by Hon’ble Union Minister of H&FW GoI.
7. AWARD:
- Regional Institute of Medical Sciences (RIMS), Imphal is the only Medical College from the entire North East to figure amongst the top 40 Medical Institutions of India for the year 2020.
RIMS is in the 28th Position in NIRF ranking 2019, released by the Ministry of Human Resource Development, Government of India.
- RIMS, Imphal was recognised as the best performing Hospital in PMJAY service in the state (2019-20) for which a Certificate of appreciation was also issued by the State Health Agency, Manipur.
- RIMS in Top 50 Best Medical Institute in India.
-RIMS, Imphal is the only Medical Institute from the entire North-East to figure among Top 50 Medical Institutes of India in the last 3 consecutive NIRF Ranking.
8. BUDGET (Rs. in crore)
|
Sl. No. |
Financial Year |
Allocation BE |
Release 2020-2021 |
|
1 |
2020-21 |
437.32 |
421.60 |
8.6 Regional Institute Of Paramedical And Nursing Sciences (RIPANS), Aizawl, Mizoram
Regional Institute of Paramedical and Nursing Science (RIPANS), Aizawl was set up by the Ministry of Home Affairs, Government of India in 1995-96 to provide Nursing, Pharmacy and Paramedical education to the people of North East including Sikkim and to maintain the pace of nursing education and nursing services with other developments medical and technological services. The institute was transferred to the Ministry of Health and Family Welfare w.e.f. 01.04.2007.
The Institute is running the following five Degree Courses and one Post Graduate Course:
|
Sl.No. |
Name of Courses |
Duration |
Intake Capacity |
|
1. |
B.Sc. Nursing |
4 years |
33 seats |
|
2. |
B.Sc. MLT (Medical Laboratory Technology) |
4 years |
33 seats |
|
3. |
B. Pharm |
4 years |
33 seats |
|
4. |
B.Sc. RIT (Radio Imaging Technology) |
4 years |
33 seats |
|
5. |
B.Optom |
4 years |
33 seats |
|
6. |
M. Pharm |
2 years |
|
|
7. |
M.Sc MLT |
2 years |
12 seats |
- No. of students newly admitted for various Courses – 194
- The total strength of students in various Courses – 683
- Total number of passed out students – 172
- About 93% of passed out students are getting placement in various Central/State Government Institutes/Departments and private establishments such as CSIR Laboratories, AIIMS, Safdurjang Hospital, NEIGRIHMS, RIMS, NIPER, GNRC, AMRI Hospital, Apollo Hospital, Birla Heart Institute, Fortis Hospital, TATA Hospital, NIT, Mizoram University, Assam Downtown University, Assam Technical University, NATCO Pharma Ltd., Torrent Pharmaceuticals Ltd., CIPLA, etc. and abroad such as Australia, USA, Canada, Ireland, England, Norway, Singapore etc. In addition, many students qualified for the All India GPAT examination conducted by National Testing Agency (NTA)
- At the onset of the Covid -19 Pandemic followed by Total Lockdown imposed by the Central Government, RIPANS made a significant contribution in the Nation’s fight against Covid 19 by preparing hand sanitizer in its own laboratory due to shortage of the same in the local market; and distributed to front line workers in the State, NGOs etc.
- The host State Mizoram had no Covid 19 Testing Laboratory at the onset of the Covid 19 Pandemic. For setting up of the Testing Laboratory, some equipment like Multi channel pipettes etc were contributed by RIPANS in addition to providing manpower expertise in various committees formed by Govt. of Mizoram.
- 48 students of Nursing, Medical Laboratory Technology and Pharmacy were working as volunteers to assist Govt. of Mizoram in the containment & prevention of Covid 19 in the state of Mizoram. These students assist in sample collection and enable opening of more vaccination Centres in the state.
- Classrooms have been upgraded and equipped with latest technology for conducting online classroom teaching and online examination. This has been achieved by using the existing resources and manpower without engaging private agencies.
- The Project of Creation of Additional Facilities at RIPANS viz. Academic Block-III , Library cum Examination Hall, Boys’ and Girls’ Hostel was completed and the buildings were handed over to RIPANS on 5.7.2019.
- Approval of Recruitment Rules of 27 new posts (including the posts of Professor, Associate Professor, Assistant Professor, Tutor, Section Officer, Accounts Officer etc.) was received from the Ministry on 22.01.2020.
- E-Tender for Civil Works of the Project of Development of RIPANS was published on 01.09.2019 (Rs. 229.46 crore). Technical bid and a financial bid were opened and recommendation to award the work to the lowest bidder at Rs. 217.97 crore was submitted to the Ministry on 5.2.2020. The estimated cost of the Project is Rs. 480.12 crore.
- Project of Development of RIPANS: Approval to award the work to the lowest bidder was conveyed by the Ministry on 04.01.2021. The work was started on 1.3.2021.
- Financial achievements :
- Approved Revised Estimate – Rs. 40.68 crore
- Amount released as on 31.3.2020 – Rs. 40.48 crore
- Expenditure incurred upto 31.3.2020 – Rs. 49.68 crore
- Capital Expenditure – Rs. 25.93 crore
- Revenue Expenditure
- Salaries – Rs. 10.39 crore
- General – Rs. 13.37 crore
(Unspent Balance of Rs. 13.71 crore as on 1.4.2019 was adjusted against GIA for the year 2019-20)
9. National Leprosy Eradication Programme (NLEP)
National Leprosy Eradication Programme (NLEP) is a Centrally Sponsored Scheme under the umbrella of National Health Mission (NHM). India has achieved the elimination of leprosy as a public health problem i.e., defined as less than 1 case per 10,000 populations, at the National level.
The NLEP aims at eliminating leprosy in each of the districts by 2030. Under the National Leprosy Eradication Programme action is taken for early case detection; complete treatment of detected cases, and to contain the onset of disease in close contacts of the index cases (persons diagnosed with leprosy).
Major steps taken so far:
- Percentage of Grade II Disability (G2D) /visible deformity in new cases has decreased from 2.41% in 2020-21 to 2.38% as on 30 th September, 2021.
- Child cases percentage has decreased from 5.76% as on 31st March, 2021 to 5.31% as on 30th September, 2021.
- In order to spread awareness about leprosy, three short films involving direct testimonials of cured leprosy patients have been developed, which are being telecast through Doordarshan channels in 18 states as per media plan.
- Sparsh Leprosy Awareness Campaigns (SLAC) were introduced and launched on 30th January, 2017 i.e., Anti Leprosy Day, to reduce stigma and discrimination against persons suffering from leprosy. Around 72%, 78% villages observed the village level meetings in Gram Sabhas on the Anti Leprosy Day during the years 2020 &2021 respectively during Sparsh Leprosy Awareness Campaigns (SLAC).
- In addition to above activities, Differential strategy guidelines for carrying out various activities under NLEP during COVID – 19 pandemic were issued to all States/ UTs in order to ensure the following:-
- Uninterrupted supply of MDT to leprosy patients during the lock-down due to COVID – 19.
- Uninterrupted DPMR services to leprosy patients suffering from physical disabilities. Besides, guidelines were issued to track the leprosy patients on treatment among the returnee migrants during COVID – 19 pandemic, and to ensure that their treatment is continued in a seamless manner at the places they migrate to. A number of such patients have been successfully tracked and treated by various states/ UTs.
10. National Center for Vector Borne Diseases Control (NCVBDC)
10.1 Malaria
- World Malaria Reports for 3 consecutive years have hailed India’s progress in achieving malaria elimination by 2030.
- Malaria cases reported in 2020 were 186532 in comparison to 338494 cases in 2019, indicating a decline of 44.9% over the year 2019. Similarly, malaria cases have declined by 17.01% and Pf cases by 18.93 % as on 31st October 2021, as compared to the corresponding period.
- In 2020 only 32 districts have Annual Parasite Incidence (API) one and above.
- In 2020, 116 districts in the country have reported ‘zero malaria cases’.
- Malaria has been made a notifiable disease in 31 states/UTs (Andhra Pradesh, Arunachal Pradesh, Assam, Chhattisgarh, Goa, Gujarat, Haryana, Himachal Pradesh, Jammu & Kashmir, Jharkhand, Karnataka, Kerala, Madhya Pradesh, Manipur, Mizoram, Nagaland, Odisha, Punjab, Rajasthan, , Sikkim, Tamil Nadu, Telangana, Tripura Uttar Pradesh, Uttarakhand, West Bengal, Puducherry, Chandigarh, Delhi, Daman & Diu, D&N Haveli and Lakshadweep).
- Till 2021, 28 states have constituted State Task force for Malaria Elimination and District Task Forces. The remaining States/UTs are in the process of constituting State Task Force and District Task Forces.
- During the last 6 years, 9.7 crore LLINs have been distributed in the high malaria endemic areas of various States/UTs .Use of LLINs has been highly accepted by the community at large and has been one of the main contributors to the drastic malaria decline in the country.
- Conducted virtual training on malaria for Additional Public Health Officers (DMO) and Vector Borne Disease (VBD) Consultants of all districts of Odisha on 15th and 18th March, 2021
- NCVBDC in collaboration with WHO and National Institute of Malaria Research organized the Malaria Microscopy Training for certification of the LTs from different States w.e.f 24-28 August ,2021 (1st Batch) and 31st August-4 September,2021 (2nd Batch) on malaria Microscopy’’ at NIMR, Delhi.
- Malaria microscopy, the gold standard for malaria elimination, has also been strengthened by National Refresher training and certification of a core group of Laboratory Technicians from different States. There are 10 L-1 and 17 L-2 WHO certified Laboratory technicians for strengthening microscopic activity and laboratory capacity building.
- In 2020-21, WHO Certified Level-1 and Level-2 Laboratory Technicians conducted total 35 Malaria Microscopy trainings in different batches at their respective States/UTs and RoH&FW (Chandigarh, Haryana, Bhubaneswar, Puducherry, Karnataka, Andaman & Nicobar Islands, Tripura, Shillong, Rajasthan and Andhra Pradesh) and 1005 participants trained under the programme.
- Setting up of health web based reporting system for the whole country on Integrated Health Information Platform (IHIP) – malaria included and mapping of high malaria-prone areas using GIS maps and hotspots
10.2 Kala-Azar
- 1152 cases have been reported during 2021 upto October in comparison to 1782 cases reported during corresponding period of 2020, reporting a reduction of 35.35% of cases. 21 deaths were reported till October, 2021.
- Till October 2021, 99% Kala-azar endemic blocks have achieved the elimination target of <1 KA case per 10,000 population at block level. 6 blocks of Jharkhand are yet to achieve the target.
- Based on the KA Independent Assessment findings, implementations of KA activities have been strengthened. High priority villages have been identified for an intensified action plan. SOPs for active case detection, outbreak management, Operational Definitions in Kala-azar Elimination Programme and guidelines for certification and awards for achieving kala-azar elimination status under the national kala-azar elimination programme have been prepared and duly disseminated to the states for necessary action.
10.3 Dengue & Chikungunya
- The number of identified Sentinel Surveillance Hospitals (SSHs) has been increased from 695 in 2020 to 713 in 2021 (till 30th November).
- Case Fatality Rate (CFR) for Dengue (deaths per 100 cases) has maintained at <1% in 2021 (till 30th November).
10.4 Japanese Encephalitis
- Out of 60 PICUs, 44 PICUs have been made functional (Assam-6. Bihar-7, Tamil Nadu-5, Uttar Pradesh -16 in 15 Dist. and West Bengal-10).
- Funds have been provided for all 10 Physical medicine & Rehabilitation (PMR) Deptts. 8 PMRs are functional (Assam-2, Tamil Nadu-1, Uttar Pradesh-3 and West Bengal-2)
- JE vaccination in Campaign mode in children (1-15 yrs.) have been completed in 297 endemic districts. 39 more districts have been identified to cover under the JE vaccination campaign in children.
- 31 districts (Assam (9), Uttar Pradesh (7) and West Bengal (15) have been covered under Adult JE Vaccination.
- 144 Sentinel sites and 15 Apex Referral Laboratories have been identified for diagnosis of JE. 487 JE IgM kits have been supplied in 2021(till 16.11.2021).
10.5 Lymphatic Filariasis
- Out of 328 (272 + 56 districts added due to bifurcation) endemic districts, 134 districts have cleared Transmission Assessment Survey (TAS)-1 and have consequently stopped Mass Drug Administration (MDA). Out of 134, TAS-2 is cleared by 121 districts and TAS-3 is cleared by 60 districts till November, 2021. During 2021 till date (30th Nov., 2021) TAS-1, TAS-2 and TAS-3 cleared 2, 2 and 7 districts respectively.
- 153 districts targeted for MDA during 2021, out of which 112 conducted MDA till date (30th Nov., 2021) [(94 DA 18 Triple Drug Therapy IDA(Ivermectin+DEC+Albendazole)].
- Regional Programme Review Group (RPRG) WHO meeting (virtual) was held on 14th -17th June, 2021.
- During 2021 till date (30th Nov., 2021), social media Tool kit was prepared and successfully used for spreading awareness about MDA rounds in the State of Karnataka, West Bengal, Odisha, Jharkhand, Chhattisgarh, Maharashtra, Telangana, Uttar Pradesh and Bihar
11. Food Safety and Standards Authority of India
- Food Safety and Standards (FSS) Act, 2006 was enacted with the objective to consolidate the laws relating to food and for laying down science based standards for articles of food as well as to regulate their manufacture, storage, distribution, sale and import to ensure availability of safe and wholesome food for human consumption . The Food Safety and Standards Authority of India (FSSAI) was established in September, 2008 under the provisions of the FSS Act as the apex authority on all matters of food safety and to ensure safe and wholesome food to consumers.
- FSSAI has constituted 21 subject specific scientific panels under Section 13 of the FSS Act, which consist of independent scientific experts, to act as the risk assessment bodies and provide their considered scientific opinion. There is also a Scientific Committee under Section 14 of the FSS Act with mandate to provide scientific opinion to the Food Authority, general co-coordination necessary to ensure consistency of the scientific opinion, and in particular with regard to the adoption of working procedures and harmonisation of working methods, of the Scientific Panels, opinion on multi-sectoral issues falling within the competence of more than one Scientific Panel and setting up working groups on issues which do not fall within the competence of any of the Scientific panels. The Scientific Committee and the Scientific Panels meet as often as required to give scientific opinions and recommend on development of food standards.
- During 2021, FSSAI continued to work towards development/revision of science based and internationally benchmarked standards of food products. During the period , FSSAI notified 16 final notifications and 14 draft notifications. Final notifications include standards/revised standards for various articles of food ; limits for incidental occurrence of khesari dal in grams/pulses; prohibition of blending of any edible vegetable oil in mustard oil; standards of fortified milk powder and tolerance limit for micro nutrients; requirement of registration and inspection of foreign food facilities etc. Draft notifications include GM food regulations, amendments to various principal regulations and two new principal regulations on Ayurveda Aahaar and Vegan Foods.
- To address concerns of Food Business Operators, facilitate ease of doing business, ensuring consumer safety and also simultaneously enhancing punishment for wrongdoers to work as deterrent, FSSAI has proposed a number of amendments in Food Safety and Standards Act, 2006. Important changes proposed in the present Act include bringing ‘export’ and ‘animal feed’ within the purview of FSSAI; harmonization of definitions with Codex and other Acts etc.; defining the role and duties of Chairperson; reviewing processes to ensure expeditious finalization of regulations; bringing more clarity to certain existing provisions; provision for reference laboratories; protecting retailer and distributor from liability in case of untampered packaged food; rationalization of penal provisions; including strengthening in certain cases; provision for creation of fund etc. The Ministry issued a public notice thereon and the response received was examined in FSSAI and comments sent to the Ministry. The amendment proposal is under process in the Ministry.
- The Food Safety and Standards Authority of India (FSSAI) had established a national network of research and academic institutions working in the area of food safety and nutrition. This network is referred as “Network of Scientific Cooperation for Food Safety and Applied Nutrition (NetSCoFAN)” and is established under the section 16(3)(e) of the Food Safety and Standards Act, 2006 which mandates the Authority to build and promote scientific co-operation, exchange of information, development and implementation of joint projects, exchange of expertise and best practices in the area of the responsibility of the Food Authority. The NetSCoFAN Website was launched by Hon’ble Minister for Health and Family Welfare on 20th September, 2021 which is an interactive platform for all the partner constituents wherein groups can easily update the status of their activities and also the NetSCoFAN secretariat can review the group activities at one place. The website covers different sections such as NetSCoFAN Groups, Resources, Login (for Groups and Admin).
- All Food Business Operators (FBOs) in the country are required to be registered or licensed under Section 31 of the Food Safety & Standards Act, 2006 to commence or carry on any food business. The Food Safety and Standards (Licensing and Registration) Regulations, 2011 regulate the procedure for grant of licence and registration to FBOs. There is an online process for issuance of licenses to Food Business Operators (FBOs). As on 31.10.2021, 1,03,684 Central Licenses, 19,74,014 State Licenses and 83,76, 312 Registrations have been issued of which 51,236 Central Licenses, 8,75,557 State licenses and 38,98,726 Registrations are active.
- Enforcement of the provisions of FSS Act and Rules and Regulations framed thereunder primarily rests with the State Governments/UTs. Food Safety Commissioners of each State/UTs along with his team of officers including Designated Officers and FSOs are responsible for regular monitoring, surveillance, inspection and sampling of food products and initiate action under penal provisions of FSS Act in cases of non-compliance. FSSAI coordinates with States/UTs through the Central Advisory Committee which meets every quarter , Video Conferencing with officials, etc.
- FSSAI has developed the State Food Safety Index to measure the performance of States on various parameters of Food Safety. Index is based on performance of States/UTs on five significant parameters, namely Human Resource and Institutional Data (weightage -20%), Compliance (30%), Food Testing Infrastructure and Surveillance (20%), Training and Capacity Building (10%) and Consumer empowerment (20%). The third state food safety index for the year 2020-21 was released on 20.09.2021. Among the larger States, Gujarat was the top ranking State, followed by Kerala and Tamil Nadu and Maharashtra. Among the smaller States, Goa came first followed by Meghalaya and Manipur . Among UTs, J&K, Andaman & Nicobar Islands and Delhi secured top ranks.
- FSSAI has launched ‘Food Safety Connect’ Mobile Application for Consumers and Food Business Operators (FBOs) on 01.10.2021. Users can now download this Mobile App from Google Play Store to stay updated with FSSAI news, important notifications or orders/advisories, events, initiatives and resource materials. Consumers can access Citizen Knowledge Hub such as Books, Videos, MythBusters. App can also be used by FBOs to file application for FSSAI Registration and access information related to notified laboratories for food product testing, Inspection Checklists, Third Party Audits, Product Standards, Food Safety Display Boards, Food Safety Mitra, FoSTaC Training, Guidance Documents etc.
- FoSCoRIS System is a digital platform for Food Safety Officers to verify compliance of food safety and hygiene standards by food businesses as per regulatory requirements. States/UTs have been asked to conduct inspections through FoSCoRIS. FSSAI has also released the offline version of FoSCoRIS, which works even in the area of limited mobile networks. The mobile app with the name ‘FOSCORIS’ can be downloaded from Google Playstore. Further, FoSCoRIS has been linked with the Food Safety Compliance System (FoSCoS) for real time flow of allocation of inspections and viewing of inspection reports by the concerned authorities and the concerned Food Business Operators. As on 16.12.2021, 1,98,026 inspections of food premises were carried out through FoSCoRIS.
- FSSAI is extending both technical and financial support to the States/UTs for strengthening the Food Safety Ecosystem in the country through MoU. Based on the proposals received from States/UTs, MoUs were executed with 24 States/UTs during 2020-21 and funds to the tune of Rs 64.66 Crore were released. For the year 2021-22, Work Proposals were received from 35 States/UTs and same have been finalized for 25 States and funds to the tune of Rs 37.87 Crore to 21 States have been released as first tranche.
- Production of blending oils containing mustard has been prohibited w.e.f. 08.06.2021. Consequently, FSSAI directed all States/UTs to carry out surveillance and targeted enforcement and it was ensured by States/UTs that all manufacturing units of Blending Edible Vegetable Oils were inspected and that such units complied with the decision. Similarly, inspections were conducted of all honey processing units for checking sugar syrups.
- To empower consumers to have an informed choice , FSSAI has mandated display of information for food service establishments having Central License or outlets at ten or more locations with effect from 01st January, 2022. The information shall include mention of calorific value (in kcal per serving and serving size) and food allergens against the food items displayed on the menu cards or boards or booklets. In addition, nutritional information also needs to be provided to the consumer upon request.
- Earlier , even small and medium manufacturers/packers of Indian Sweets and Snacks & Savouries were required to take Central License under proprietary food products which was not only costly but also entailed onerous compliances for the said category. To ease the licensing/registration for such small and medium food businesses, it was decided to assign the Food Product Category 18 in FoSCoS under ‘General Manufacturing Kind of Business’ and FBOs could obtain Central / State license or registration based on their production capacity and turnover.
- Screening of all Food and Beverages related advertisements appearing in various print and electronic media is a big challenge. Further, it is difficult for limited manpower with FSSAI to take recourse on all such cases wherein advertisements are found misleading and also to keep regular watch on the same. Therefore, FSSAI signed MoA with Advertising Standards Council of India (ASCI), a self-regulatory voluntary organization , for exclusive tracking, tracing and evaluation of all Food and Beverages advertisements appearing across various media which could be potentially violating provisions of FSS Act, Rules and Regulations made thereunder and for its recommendation to FSSAI for further investigation.
- Presently, FSSAI number is to be displayed compulsory on packaged food labels. To enable consumers to know the FSSAI number of the service/ product provider , FSSAI has mandated mentioning of License/ Registration number on receipts/invoices /cash memo/ bills etc. by food businesses on sale of food products from 01.01.2022.
- During 2021, 43 more food laboratories have been recognised /notified and 09 food testing laboratories de-notified under Section 43 (1) of Food Safety and Standards Act, 2006 by FSSAI. This has raised the total number of notified food laboratories from 188 to 222 till date. All FSSAI notified labs are NABL accredited. In addition, 04 State Food testing laboratories (SFTLs) are also functioning under transitory provisions of Section 98 of FSS Act which will be notified by FSSAI upon obtaining accreditation from NABL, expected soon.
- During the year, FSSAI has inaugurated National Food Testing Laboratory (NFL) at JNPT , Mumbai under PPP mode while another NFL at Chennai Port Trust under PPP mode is likely to be ready by January, 2022 . These are in addition to FSSAI’s existing National Food Testing Laboratories at Ghaziabad and Kolkata. Ghaziabad lab has been established under PPP mode.
19. Under a Central Sector Scheme for upgradation of food testing infrastructure in States , a grant of Rs. One Crore has been released during the year to 2 States/UTs for procurement of Basic/High-end Equipment and setting up of Microbiology testing facilities (with CAMC and manpower) towards upgradation of 2 State Food Testing Laboratories (SFTLs). With this, a total Grant-in- aid of Rs.313.98 Crore has been sanctioned/released to 29 States/UTs for upgradation of 39 State Food Laboratories, including setting up microbiological laboratories in 25 SFTLs.
20 During the year , a grant of Rs. 7.60 Crore (approx.) has been released to three referral labs viz. Punjab Biotechnology Incubator (PBTI), Mohali; Central/ Referral Food Laboratory, Pune ; ICAR- CIFT, Kochi and CFRA- NIFTEM, Sonipat towards the procurement of High-end Equipments.
21. 70 more Food Safety on Wheels (FSWs), each costing Rs. 37 lakhs, alongwith grants for fuel and consumables to the extent of Rs. 10 lakh/per year for two years, have been sanctioned to 11 States/UTs during 2021. This has raised the total number of FSWs sanctioned from 90 to 160 across the country covering 33 States/UTs. Each FSW can conduct 64 tests. These mobile food testing labs are being used by States/UTs for food testing, training and awareness generation , particularly in remote areas.
22. FSSAI is implementing a Sample Management System (SMS) across the country under which cold chain facilities for storage and transportation of food samples are being provided to food safety departments of States/UTs. FSSAI has, till date, provided 796 Compact Cabinets, 797 Vehicle Mounted Mobile Freezer Boxes, 2545 Portable Chill Boxes and 2545 Backpack Style Bags to 33 States/UTs. SMS would be provided to remaining States/UTs subject to readiness of the State/UT Governments. This would ensure that the food samples taken by the Food Safety Officers reach food testing labs under cold chain maintaining the integrity of the samples.
23. FSSAI is in the process of conducting a gap analysis of state food testing labs in terms of test equipment, manpower, testing scope etc. This would help FSSAI in strengthening food testing laboratories in India. The job has been awarded to a duly selected firm and the final report is likely to be available by March, 2022.
24. FSSAI conducted a PAN- India Edible Oil Survey in August, 2020 on 15 different types of edible oils. The salient findings of the survey report have been shared with the Chief Secretaries/Food Safety Commissioners of the concerned States/ UTs for appropriate action. Further , the Secretary, Ministry of Agriculture &FW and Ministry of Food Processing Industries were informed to take appropriate remedial measures for the non- compliance observed in the Survey with respect to the presence of Aflatoxins, Pesticide Residues and Heavy Metals in edible oils.
25. The Pan-India survey on the Milk Products (Khoya/Paneer/Chhana/ Khoa based & Paneer based sweets) was conducted in November 2020 during festival period (Diwali) in the country to assess the quality of Milk products. The report of the survey is under the process of finalization for future course of action.
26. The PAN- India baseline survey on industrially produced trans-fat and acrylamide content was conducted from 29th June – 2nd July, 2021, in 6 selected food categories. The trans- fat was analyzed in 6 pre- defined food categories (Sweets, Toppings and Chocolates; Fried Foods; Bakery and Confectionary products; Frozen Foods; Composite Foods and Oils, Vanaspati, Shortenings and Margarine) and acrylamide was analysed in three categories out of the selected 6 categories (Fried Foods; Bakery and Confectionary and Composite Foods). The report of the PAN- India Survey was released by the Hon’ble Minister of Health & Family welfare on 20th September, 2021. The results revealed that only 3.14 %( 196 samples) contained trans-fat exceeding 2%. The findings of the Survey revealed that the Food Processing Industry is positive about FSSAI’s regulation for eliminating the industrially produced trans-fats in foods by 2022.
27. As per Section 25 of the Food Safety & Standards Act, 2006, all imports of articles of food are subject to the provisions of the Act. It stipulates that no person shall import into India any article of food in contravention of the Act or any rules and regulations made thereunder. Exercising the power of the Act, the Central Government, on the recommendations of the Food Authority, notified the FSS (Import) Regulations, 2017 on 9th March, 2017.
28. Food import into the country is being regulated at 150 rationalised points of entry . FSSAI earlier had its presence at 22 points of entry under 6 locations namely Chennai, Kolkata, Mumbai, Delhi, Kochi and Tuticorin . However, during the period, FSSAI has brought under direct control new food import entry points and operationalized offices at Krishnapatnam, Mundra, Kandla, Hyderabad, Vishakhapatnam, Ahmedabad & Bengaluru . With this, total 53 Points of Entry of food import are now under direct control of FSSAI officials. At other 97 points of entry, Customs officers have been notified as Authorised Officers to regulate the clearance of food consignments as per the norms prescribed by FSSAI for which they have been provided requisite training. An online training portal has been developed for Customs officers which can be accessed 24×7.
29. FSSAI has its own food import clearance system (FICS) which is an online system, integrated with the Customs’ ICE-GATE (Indian Customs Electronic Commerce/Electronic Data interchange (EC/EDI) Gateway) under SWIFT (Single window interface for facilitating trade). Customs Department implements the Risk Management system (RMS), i.e. selective sampling and testing of food articles, under SWIFT in consultation with FSSAI. FSSAI has set the parameters for RMS to be applicable on imported food items. RMS is being applied in ICEGATE before sending the consignment/bill of entry (BOE) in FICS. FSSAI conducts 3 tier scrutiny – documents, visual inspection and food sample testing in labs before issuing No Objection Certificate or Non-Conformance Report to Customs.
30 . During the period, FSSAI has notified FSS (Import) Amendment Regulations on 3.11.2021 with provision for licensing/registration of foreign food facilities exporting food to India and inspection of the same. Further, to facilitate imports of pulses and edible oils to ensure timely processing and clearance, it was clarified that importers of pulses and edible oil may carry out advance filing of Bills of Entry in FICS of FSSAI . Authorised Officers were asked to facilitate and carry out the food import clearance process on priority without delay and if required, scrutiny/visual inspection/sampling may be carried out even on weekends also to expedite the process. Average clearance time has reduced to 110 hours from 125 hours in previous year.
31. FSSAI has carried out Integration of the SEZ Online system of NSDL operational at SEZ with Food Import Clearance System (FICS) of FSSAI. This has been implemented on 01.09.2021. The Bills of Entry for food import filed at notified SEZ PoEs are now seamlessly transmitted online to FSSAI for food import clearance purposes.
32. FSSAI had introduced Food Safety Training and Certification (FoSTaC) Programme in May 2017 with 19 short duration programs of 4-12 hours for different kinds of food businesses with focus on good hygiene and manufacturing practices based on Schedule IV of Food Safety & Standards Regulations, 2011. Under this initiative, more than 3.17 lakh food handlers have been trained during 2021 through 261 Training Partners and more than 2100 Trainers. Besides, through 793 training programmes, FSSAI has provided basic training to more than 27,000 Street Food Vendors . Further, FSSAI had launched a 2 hour online training programme for food business operators exclusively on COVID-19 preventive guidelines. During the year, 81 Covid-19 trainings were conducted and more than 1500 persons were trained. The training is being delivered online/offline by trained and certified trainers. Further, during the period, induction/refresher courses have also been conducted for more than 200 regulatory personnel
33. FSSAI signed an MoU with the Ministry of Food Processing Industries on 1.10.2021 to support micro-level food entrepreneurs and Farmer Producer Organizations (FPOs) to improve the food safety and hygiene standards of their food businesses. Food handlers of these micro level food processing units will be provided training on understanding of good hygiene, food testing process and other regulatory requirements. Support will also be provided in obtaining FSSAI license and registration.
34. The Eat Right India movement has been launched by FSSAI. It is a nation-wide campaign with focus on preventive and promotive healthcare through social and behavioural change. It is based on three key themes- Eat Safe, Eat Healthy, and Eat Sustainable. It integrates food safety, public health nutrition, and environmental sustainability using a mix of regulatory, enabling , and capacity building approaches towards various interventions for food businesses and consumers. Multifarious initiatives are being undertaken under this initiative on a regular basis. It is overseen by an Inter-Ministerial Steering Committee.
35. To scale up various Eat Right initiatives across States/UTs, a competition known as ‘The Eat Right Challenge’ for districts and cities to recognise their efforts in adopting and scaling up various initiatives has been launched. 188 cities and districts from States/UTs all over the country are participating in this challenge which is to culminate by December, 2021.
36. FSSAI has also launched the Eat Smart Cities Challenge, in partnership with the Smart City Mission under the Ministry of Housing and Urban Affairs, with an aim to create an environment of right food practices and habits in India’s smart cities. 109 smart cities have participated in this challenge.
37. FSSAI has launched the Eat Right Research Awards and Grant to have a wider collaboration with various academic and research institutions for encouraging and recognising high-quality research in the area of food safety and nutrition with a view to update or upgrade food formulations and/or technologies (as per the standards defined); awareness and availability of more variants of safe and healthy food. The applications were invited till 15th December, 2021.
38. FSSAI through its social media platforms is spreading awareness regarding healthy food options. FSSAI promotes a variety of whole grains ranging from wheat/rice to millets and other indigenous grains for better nutrition. A series of efforts are being undertaken including “Recipe Ravivaar” on social media to promote seasonal vegetables and indigenous food grains. Various scroll messages on Eat Right India and necessary requirements related to licensing and registration process for FBOs were telecast on DD News, DD Kisan and DD Regional Kendras.
39. Various resource materials and a number of recipe books with simple recipes have been released during the year. These books are :
- ‘Ghar ki Rasoi Tasty Bhi, Healthy Bhi’: It includes recipes developed in-house by the employees of FSSAI.
- ‘Indi-genius Recipes Book’: Recently, the UN General Assembly adopted a resolution sponsored by India to declare the year 2023 as ‘International Year of Millets’. With this backdrop, a healthy recipe contest named “Indi-Genius Food Challenge”, was launched on the occasion of 75 years of India’s independence. The challenge not only sensitized people to learn about the benefits of indigenous millets and other ingredients but also encouraged them to use them innovatively. This book contains 75 winning recipes.
- ‘Plant Protein Breakfast recipe book’: It compiles recipes with emphasis on plant-based sources of protein.
- ‘History and Food’: Indian cooking derives from a 5000-year-old timeline. There are various food items that are associated with historical events. This book provides the story behind the evolution of different dishes and are presented in the way as submitted by the participants.
- ‘National Low Salt Cooking Challenge’: Recipes collected during the competition on ways to cook common foods using less salt and without compromising the taste of food were also compiled.
These e-books are available on the website for easy access by the public.
40. Further, to celebrate ‘Azadi Ka Amrit Mahotsav’ to commemorate 75 Years of India’s Independence, FSSAI is organizing “Eat Right Walkathon and Eat Right Melas” in 75 cities across India. The events aim to propagate the message of Safe, Healthy and Sustainable diets. There is focus on millets and fortification during Melas.
41. Eat Right Tool Kit- It is a kit for use by frontline workers to use for sensitization of the community they are working with. TOT for National and State trainers for the ‘Eat Right Toolkit’ programme has been jointly completed by FSSAI, NHSRC and VHAI for all States/UTs.
42. To improve the micronutrient status of the population, FSSAI over the last one year has coordinated and partnered with Ministry of Health and Family Welfare, Department of Food and Public Distribution, Ministry of Women and Child Development, Ministry of Education, NITI Aayog and development partners to scale up food fortification in the country. To ensure quality of the products, regular training of various stakeholders were carried out through online and offline mode.
Capacity building program on implementation of Staple Food Fortification in Anganwadi Centres for Delhi was done. Sensitization workshops with consumers’ cooperatives such as Kendriya Bhandar, NAFED were also conducted with their sales staff to enable them to promote fortified staples in the stores and inform consumers about the health benefits of +F products. Multiple national state level webinars were done to scale up food fortification. A national webinar was jointly organized by FFRC, FSSAI, and GAIN on World Milk Day 2021 to deliberate on how this nutritious food can be further improved through fortification to impact lives positively. Extensive efforts have been made to generate awareness amongst common people through articles on fortified foods and their health benefits in leading magazines/ online media platforms.
43. Short films on Rice fortification in all regional languages were created and disseminated to State officials and DoFPD for consumer awareness. IEC activities on collecting community feedback on consumption of fortified rice in three districts of Madhya Pradesh under ICDS scheme was done. Recently on the occasion of 75th year of Independence, Hon’ble Prime Minister announced fortified rice to be distributed under various government schemes by 2024. To scale-up rice fortification FSSAI is working towards converging and synergising the efforts of all stakeholders in the success and sustainability of the program through strong public-private partnerships (PPPs) with engagement of consumers, civil society, donors, etc.
44. A radio campaign was done across 40 cities in India starting from 19th July to 20th August 2021 to sensitize the public on fortified milk and popularizing the + F logo. Efforts have been made to encourage dairies to amplify the campaign so as to reach a large number of consumers. Fortified foods were promoted through a show on the channel Zee Zest, The Grand Trunk Rasoi with Chef Harpal Singh Sokhi who prepared recipes using fortified foods. Pamphlets and sticker distribution via online grocery stores was done to further raise awareness among consumers.
45. FSSAI continued to function as the National Codex Contact Point (NCCP) of India, and participate actively in the Codex work for development of international standards that are fundamental to ensuring safety and fair practices in international trade of food products.The meetings of the subsidiary bodies of the Codex Alimentarius Commission took place virtually. The Indian delegation attended various virtual meetings during the year . India made specific proposals and/or ensured that India’s concerns were addressed.
46. FSSAI is regularly working to explore the possibilities of developing interactions and collaborations with various countries which will help in formulating technical standards of food, sanitary, safety etc. as per international norms. FSSAI has entered agreements with nine counterpart agencies of various countries for scientific and technical cooperation which promotes consistency, among others, in domestic and international standards. It also regularly conducts/participates in meetings with various countries at different levels, discussing potential areas of collaboration and understanding and implementing the best practices. Further, the bilateral meetings/interfaces are helping the countries to better understand the FSSAI mandate.
47. The sanctioned strength of FSSAI was increased from 356 to 824 in the year 2018. First Phase recruitment process was initiated in 2019 for 288 posts at various levels which has already been completed. More than 250 selected candidates have already joined against various posts. At present , there are 563 persons in position in the Authority on direct recruitment/deputation/contract basis. The recruitment process for 37 Deputy Director and Joint Director level posts is at an advanced stage and it is expected that these posts will be filled up shortly. In addition, FSSAI has also initiated a recruitment process for vacant posts in a few more categories . The advertisement for these posts was published on 2.10.2021 .
12. Status and key achievements of National AIDS Control Programme for the Year 2021
- The Government of India is currently implementing the National AIDS Control Programme (NACP)-Phase IV (Ext.) as a fully funded central sector scheme to respond to the HIV/AIDS epidemic in the country. Under the programme, comprehensive services across HIV prevention-testing-treatment-retention are being offered through 1,471 non-government organizations/community-based organizations targeted interventions, around 34,500 HIV counselling and testing/screening facilities and 645 ART Centres.
- During January 2021 to November 2021, approx. 80 lakh people from high-risk group and bridge population were covered under the programme. In the same duration, around 3.74 crores screening and testing for HIV was done that included around 1.64 crore pregnant women with positivity of 0.51% (general individuals) and 0.05% (pregnant women). As of Nov 2021, around 15.21 lakh HIV infected people were on ART including around 1.06 lakh in the private sector.
- National AIDS Control Programme (NACP) Phase IV (Extended) has ended on 31st March 2021 (FY 2017-21). For continuation of the NACP for a period of 5 years from 1st April 2021 to 31st March 2026, Expenditure Finance Committee (EFC) Memo with an outlay of Rs.15,471.94 has been appraised in a meeting held under the Chairmanship of Finance Secretary and Secretary (Expenditure) dated 21st September 2021. During the current Financial Year (2021-22), BE for National AIDS Control Organization (NACO) is Rs.2900.00 crore, out of which an expenditure of Rs.1445.75 crore has been incurred upto 29th December 2021.
- The HIV/AIDS epidemic in India continues to be at a low level with adult (15-49 years) HIV prevalence of 0.22% in 2020 (HIV Estimates 2020). The country is estimated to have around 23.19 lakh people living with HIV/AIDS in country. Overall, the impact of the programme has been significant with around 48% decline in new HIV infections since 2010. Similarly, estimated AIDS related death has declined by 88% since 2010. As per UNAIDS 2020 data, the global average for decline in new infections and AIDS related deaths since 2010 has been 31% and 47%respectively.
- In terms of First 90, around 76% (17.75 lakh) of estimated PLHIV were aware of their HIV status in 2019-20 which has increased to 78% (18.10 lakh) in 2020-21. Among the PLHIV who know their HIV status, around 84% (14.86 lakh) in 2019-20 and 83% (14.94 lakh) in 2020-21 were on ART (Second 90). In 2019-20, among PLHIV on-ART, who were tested for viral load, 84% were virally suppressed which increased to 85% in 2020-21 (Third 90). As evident, progress on the first and third 90 has been significant in 2020-21 vis-à-vis 2019-20.
- A United Nations General Assembly High-Level Meeting (UNGA HLM) on HIV/AIDS was held from 8th to 10th June 2021 in New York. The meeting was conducted in hybrid mode – both in-person and virtual. The Hon’ble Union Minister of Health and Family Welfare led the Indian delegation. A Political Declaration which encompasses the vision and approach toward progress of 95-95-95 targets and 2030 goal was adopted during the high-level meeting.
- ‘THE HUMAN IMMUNODEFICIENCY VIRUS AND ACQUIRED IMMUNE DEFICIENCY SYNDROME (PREVENTION AND CONTROL) ACT’ has been enacted and implemented to legally protect all people infected and affected with HIV/AIDS from any social, medical, educational, employment and financial discrimination
- Till date, NACO has signed 18 MoUs with key Ministries/ Departments to catalyse HIV/AIDS response under their mandate.
- Roll out of Dolutegravir based Regimen: Dolutegravir based Anti-Retroviral regimen has been introduced under NACP in view of lesser side effects, better viral suppression, and high threshold for development of resistance. Currently more than 9.78 lakh patients have been transitioned to Dolutegravir regimen which is approx. 70% of the total PLHIV on ART under the programme.
- With the adoption of the ‘Test & Treat’ policy, all People Living with HIV (PLHIV) are eligible for ART initiation, irrespective of their CD4 counts or WHO staging. Under the current system, a PLHIV is required to make multiple visits to ART centers before ART initiation, particularly for lab investigations. Considering the issue, a special algorithm was released for all ART centers to be followed for ART initiation among PLHIV. The Algorithm considered that same day/rapid initiation of ART is facilitated, wherever possible. This initiative will help to avoid unnecessary delay in ART initiation and inconvenience to PLHIV leading to some patients becoming LFU even before the initiation of ART.
- Under the banner of Azadi ka Amrit Mahotsav, NACO has conducted three major awareness campaigns on HIV/AIDS, Tuberculosis and Voluntary Blood Donation. To align with the 75th year of Independence, it was envisioned to fruitfully engage 75 schools and 75 Red Ribbon Clubs (RRCs) in phased manner throughout the year to spread awareness about HIV, TB and Voluntary Blood Donation Day.
- NACO has initiated its Instagram account during the launch of 1st phase of New India@75 under Azadi ka Amrit Mahotsav i.e. August, 2021. With this NACO is now present on four major social media platforms i.e. Facebook, Twitter, Instagram & Youtube as @NACOINDIA. NACO has carried out several promotional campaigns on its Social Media Platforms throughout the year such as all phase I of India@75, phase II of India@75, World AIDS Day and several others.
- The ‘Grand Finale of the Red Ribbon Quiz Competition’ was organized by National AIDS Control Organisation (NACO) on 12th January 2021 on National Youth Day. The objective of the quiz competition was to generate awareness among youth on HIV/AIDS and related issues. The ‘Red Ribbon Quiz Competition’ was planned across the country starting at District level, engaging 518 districts, to select teams for State level Competitions. The winners from these States level Competitions then participated at regional levels. The winning team from the four regions of the country then participated in the ‘Grand Finale of the Red Ribbon Quiz Competition’.
- National operational guidelines for ART services 2021 and National Technical Guideline on Anti-Retroviral Treatment 2021 were released on 1st December 2021.
- NACO has rolled out Sampoorna Suraksha Strategy (SSS), a new immersion learning model to provide a comprehensive HIV/STI services package for HRGs and other “At-Risk” population in a stigma-free environment.
- With constant efforts and guidance from NACO, all 64 public sector viral load laboratories have become functional and are performing routine viral load testing.
- NACO has introduced Quality Assurance Scheme (QAS) for Stand Alone Integrated Counselling and Testing Centres (SA-ICTCs) as a measure to maintain quality of testing being performed at these centres. With continuous support and efforts from NACO, some SA-ICTCs have been certified achieving the highest level of quality.
13. E-Health
National telemedicine services
The National Telemedicine Service “eSanjeevani” is a digital health initiative of the Ministry supports two types of teleconsultation services-Doctor-to-Doctor (eSanjeevani) and Patient-to-Doctor (eSanjeevani OPD) Tele-consultations. eSanjeevani was rolled out in November 2019 as an important component of the Ayushman Bharat Health and Wellness Centre (AB-HWCs) programme. It aims to implement tele-consultation in all the 1.5 lakh Health and Wellness Centres in a ‘Hub and Spoke’ model, by December 2022. NHM in States identify and set up dedicated ‘Hubs’ in Medical Colleges and District hospitals to enable tele-consultations services to ‘Spokes’, set up at SHCs and PHCs.
In wake of COVID 19 Pandemic, on the 13th April 2020, the MoHFW rolled out ‘eSanjeevaniOPD’ first of its kind to facilitate online health services to patients directly in the confines of their home at no cost to ensure continuity of care.
eSanjeevani has completed around 2 crore consultations. Over 1, 00,000 patients are seeking health services on a daily basis in 35 States/UTs. The top ten States which have registered highest consultations through eSanjeevani and eSanjeevaniOPD platforms are Andhra Pradesh (7665939), Karnataka (3281070), Tamil Nadu (1744038), Uttar Pradesh (1537339), West Bengal(1262330), Bihar(632474), Gujarat (590564), Madhya Pradesh (577513), Maharashtra (574457), Uttarakhand (345342),
Ayushman Bharat Digital Mission (ABDM): In year 2019, Ministry of Health and Family Welfare released National Digital Health Blueprint (NDHB) as an architectural framework for effective implementation of Digital Health interventions.
With a vision to create a national digital health ecosystem as proposed in NDHB, on 15th August 2020, Hon’ble Prime Minister Shri Narendra Modi announced the launch of the National Digital Health Mission (now known as Ayushman Bharat Digital Mission) in six union territories (Andaman & Nicobar Islands, Chandigarh, Dadra & Nagar Haveli and Daman & Diu, Lakshadweep, Ladakh and Puducherry) on pilot basis. Three key registries of NDHM namely Health ID, Health Professional Registry (HPR), Health Facility Registry (HFR) and digital infrastructure for data exchange have been developed and implemented in these UTs.
On 27th September, 2021, the Hon’ble Prime Minister announced the nationwide rollout of the Ayushman Bharat Digital Mission (ABDM)(earlier known as National Digital Health Mission) with aims to develop the backbone necessary to support the integrated digital health infrastructure of the country.
14. National Centre for Disease Control (NCDC)
National Centre for Disease Control (NCDC) has its headquarters in Delhi and has 8 branches located at Alwar (Rajasthan), Bengaluru (Karnataka), Kozhikode (Kerala), Coonoor (Tamil Nadu), Jagdalpur (Chhattisgarh), Patna (Bihar), Rajahmundry (Andhra Pradesh) and Varanasi (Uttar Pradesh).
The technical Centres/Divisions at the headquarters of the institute are:
- Integrated Disease Surveillance Programme (IDSP)
- Division of Epidemiology
- Division of Microbiology
- Division of Biotechnology and Viral Hepatitis
- National Program for Surveillance of Viral Hepatitis
- Division of Parasitic Diseases
- Centre for Arboviral & Zoonotic Diseases
- Division of Zoonotic Disease Programme,
- Centre for Environmental & Occupational Health, Climate Change & Health
- Centre for Non-Communicable Diseases
- Integrated Disease Surveillance Programme (IDSP)
IDSP covers all states and UTs with the objective to strengthen/maintain decentralized laboratory-based IT enabled disease surveillance system for epidemic prone diseases and to monitor disease trends to detect and respond to outbreaks in early rising phase through trained Rapid Response Team (RRTs). IDSP has also been coordinating the overall surveillance activities in India regarding CoVID – 19 pandemic. A total of 470 outbreaks of epidemic prone diseases like Kyasanur Forest Disease, Crimean-Congo Haemorrhagic Fever, Seasonal Influenza A (H1N1), Anthrax, Leptospirosis, Scrub Typhus etc. have been detected by IDSP (till 19th Sept’ 2021). CSU, IDSP has been assisting the States/UTs in their epidemiological investigation and containment. A near real time, web enabled electronic health information system called Integrated Health Information Platform (IHIP) was rolled out on pilot in 7 States namely Andhra Pradesh, Himachal Pradesh, Karnataka, Odisha, Uttar Pradesh, Telangana, and Kerala on 26th Nov’ 2018. Till date, 11 States/UTs have completely transitioned to the IDSP-IHIP platform. Transition of the rest of the States/UTs is expected to be complete by 31st March’ 2022.
- Epidemiology Division
Various activities coordinated by the division are:
- Public Health capacity building: 3X3 Basic Epidemiology Training for Frontline Public Health Workforce undertaken and completed in Uttarakhand, while ongoing in J&K, Rajasthan, Arunachal Pradesh.
- Outbreak investigations & Disaster management: actively involved in the COVID-19 Pandemic Response and developed technical documents, supported epidemiological investigation of clusters, risk communication and community engagement, central RRTs- Maharashtra, Punjab, Chhattisgarh, Uttar Pradesh, J&K, Rajasthan, Madhya Pradesh, Patna, Mizoram, publishing a Special COVID-19 Issue of NCDC Newsletter and CD Alerts on COVID-19 and COVID-19 associated Mucormycosis. The division also played a role in Special Surveillance activities during mass gatherings at Haridwar Kumbh mela, to detect and manage outbreaks.
- India Epidemic Intelligence Service (EIS) Programme: The NCDC EIS programme graduated 4 cohorts of 41 officers, and ongoing is7th cohort with 8 officers and started a new cohort of 12 officers. In the ongoing COVID-19 pandemic, EIS officers were involved in COVID 19 response in Punjab, UP, Kerala, Odisha, MP, Delhi, Maharashtra, Arunachal Pradesh and surveillance of communicable diseases during Kumbh Mass gatherings.
- AMR: Epidemiology division provides technical support to the National Programme for Containment of Antimicrobial Resistance and Consumption. A two-day training and review workshop was organized by NCDC, Delhi on 29-30 September 2021 and the division is also coordinating point prevalence surveys of antimicrobial consumption across 25 NAC-NET sites in collaboration with WHO.
International Health Regulations
NCDC is designated National Focal Point for India. India has declared itself IHR compliant in July 2016. Functions of NFP include: capacity building for IHR(2005) in the country, review progress of IHR implementation by using WHO IHR monitoring tool and share with WHO annually, coordination and communication with WHO, NFP of other countries and local stakeholders for event verification, notification, contact tracing(TB), etc.
INSACOG:
- The Indian SARS-CoV-2 Genomics Consortium (INSACOG) is a national multi-agency consortium of Genome Sequencing Laboratories (RGSLs) laboratories established by the Government of India on 30th December 2020. Initially, this consortium had 10 laboratories. Subsequently, the scope of laboratories under INSACOG was expanded and at present there are 38 laboratories across India under this Consortium which monitor the genomic variations in SARS-CoV-2.
- The network carries out whole genome sequencing of SARS-CoV-2 virus across the nation, aiding the understanding of how the virus spreads and evolves, and provides information to aid public health response.
- Presently, under the sentinel surveillance strategy, adapted from WHO nearly 300 sentinel sites are identified to adequately represent the geographic spread across more than 700 districts of India. RTPCR positive samples are sent from each sentinel site for Whole Genome Sequencing.
- Additionally, surge surveillance for districts with COVID19 clusters or those reporting a surge in cases is functional.
- In view of the threat of new variants entering from abroad a Point of Entry (POE) screening is being conducted from Dec 2020 onwards. In view of omicron threat POE screening has been scaled up by identifying at risk countries by MOHFW.
- Alongside, proposals for sequencing of sewage samples and clinical samples is also under review.
Status of Variants and Sequencing as on 29 Dec 2021:
- Total samples sequenced: 1,11,587
- INSACOG samples with PANGO lineage assigned: 94,169
- Total VOCs: 66832
- Total Omicron cases: 579
- Division of Microbiology
Respiratory Viruses Lab:
- Supported lab diagnosis of H1N1 & other influenza subtypes, SARS-CoV-2 and teratogenic viruses (Rubella, CMV, HSV-1&2).
- Surveillance for H1N1: NCDC has a network of around 12 labs for regular influenza surveillance. RT-PCR for more than 1.10 lakh samples for COVID-19 from various states till date.
- Participated in WHO EQAS for SARS-CoV-2 and Influenza and achieved 100% score. Training of lab personnel from different institutions in lab procedures on COVID-19 testing.
National programme on AMR containment:
Coordinating the “National Programme on Antimicrobial Resistance Containment” under which a network of State Medical college laboratories (NARS-Net) across the country are being strengthened in a phased manner for AMR containment activities. This programme is one of the sub-schemes under the Central Sector Umbrella Scheme of NCDC and currently includes 35 state medical college labs & Hospitals in 26 states/UTs.
- Induction and refresher online training on use of WHONET software for entry and analysis of AMR surveillance data and antibiogram preparation has been conducted for all the sites.
- AMR National Reference Laboratory (NRL) at the CBDDR conducted EQAS and AMR Alert identification for 29 network sites for the quarter 1 and quarter 2 of the year 2021. Molecular testing methods have been initiated at the AMR NRL for confirming AMR alerts and a whole genome sequencing facility for AMR detection and surveillance has been established in July 2021. AMR surveillance data for the year 2020 has been analysed and submitted to Global AMR Surveillance System (GLASS).
Enterovirus Division:
Acute Flaccid Paralysis (AFP) Surveillance and Environmental Surveillance for Polioviruses (EPS)
Stool specimens from acute flaccid paralysis (AFP) cases are received from Delhi, Haryana, Uttarakhand, some parts of Uttar Pradesh and rarely from Madhya Pradesh and Rajasthan. Virus isolation and Real time PCR for identification of wild polio viruses, vaccine derived polio viruses and other enteroviruses is done on all AFP and sewage specimens.
The laboratory is WHO accredited for testing of Measles and Rubella samples (IgM antibody detection by ELISA).
Diagnostic Support for investigation of other viruses such as Parvo Virus B-19, Varicella Zoster Virus, Mumps Virus, Adeno Virus, Enteroviruses and Epstein Barr Virus.
Centre for Aids and Related Diseases (CARD)
- External Quality Assessment Schemes (EQAS) for HIV serology, HIV DBS testing and CD4/CD4% T- Lymphocyte count – achieved 100% concordant results.
- HIV sero-status confirmation through Western Blot test of indeterminate and discordant serum samples from State Reference Laboratories (SRLs).
- Counseling and testing in ICTC for walk-in clients for HIV and syphilis and training to SRLs and EQAS panel preparation for HIV serology for distribution to 13 SRLs and 424 ICTCS.
- Kit quality testing of HIV, HBV and HCV kits used under NACO programme as part of Consortium of NRLs (National Reference Laboratories).
- Division of Biotechnology and Viral Hepatitis
The division provides molecular diagnostic services, molecular epidemiology, specialized training and applied research on various important epidemic-prone diseases of public health importance
- The Covid-19 testing facility of the Biotechnology Division during this period tested 2,10,904 samples using the Roche Cobas 6800 automated system.
- The division has also established a Genome sequencing facility and has till 30th of Sept. 2021, sequenced 19,200 SARS-CoV-2 samples on Illumina NextSeq 550 platform. All of the sequences are continuously being uploaded on the GISAID database.
- 270 (Covid positive and Covid negative) samples sequenced using the RVOP kit on Illumina NextSeq 550 platform to identify co-infection or presence of other respiratory viruses.
- Using Sanger technology the Division has sequenced >200 samples for target specific sequencing for other respiratory viruses and also identified using BLASTn.
- 45 sequences of Dengue targets, sequenced in the division were also uploaded on the NCBI Genbank database.
- Biotechnology students were also trained on various molecular techniques in the division with four students completing their Masters dissertation.
- National Program for Surveillance of Viral Hepatitis
Surveillance of Acute Viral Hepatitis: Activity for surveillance of acute viral hepatitis initiated in 2021 based on Operational guidelines and strategies developed by a technical resource group composed of public health experts, microbiologists, clinicians, program managers from across the country. Enhanced Case Reporting being done in all 15 sites strengthened under the program.
Training Module developed and capacity of human resource involved in the activity built
Surveillance of Chronic Viral Hepatitis-Integration with population level surveys/ programs
- National Family Health Survey (NFHS)-4: Integrated with ICMR, IIPS Mumbai and ICMR NARI Pune for inclusion of markers for hepatitis B and C First ever nationally representative data for seroprevalence of both hepatitis B and hepatitis C obtained
- Integration with HIV Sentinel Surveillance (HSS) of National AIDS Control Program (NACP): Advocacy with NACP for Inclusion of bio-markers of hepatitis B and C in HSS utilising existing machinery of HSS with minimum financial implications. Current round of HSS plus ongoing.
- Integration with National Viral Hepatitis Control Program (NVHCP): Establishing linkages and mechanisms for follow-up of individuals detected positive on screening for hepatitis B/C for counselling, confirmatory testing and linkages to care and support services to high risk groups, prison inmates and pregnant women and their newborns.
- Department of Parasitic Diseases (DPD)
Deals with the activities related to Neglected Tropical Diseases namely Soil Transmitted Helminthiasis (STH), Guinea worm Disease and Lymphatic Filariasis. NCDC functions as the National Nodal Agency for implementation and monitoring of the National Guinea Worm Eradication Programme. The Department also functions as National Nodal Agency for prevention and control of STH in the country and has been continuously monitoring the STH burden in the country through periodic prevalence assessment surveys.
- Department compiled and submitted data on STH prevalence for 36 districts in 6 states/UTs namely Karnataka, Kerala, Maharashtra Jammu, Chandigarh and Himachal Pradesh.
- The Department is supporting filaria elimination activities through capacity building of medical & paramedical health personnel in filariology.
- Centre for Arboviral & Zoonotic Diseases
Deals with the Zoonotic diseases of public health importance including outbreak prone and emerging infectious diseases mainly Plague, Rabies, Kala-azar, Arboviral infections (Dengue, JE, Chikungunya, Zika virus & CCHF) Toxoplasmosis, Brucellosis, Leptospirosis, rickettsiosis, hydatidosis, neurocysticercosis, SARS Cov2, and Anthrax. The role of division is primarily to provide laboratory evidence by conducting special and reference level tests which are not available at most of the institutes or medical colleges in India.
- Division of Zoonotic Disease Programme
Nodal agency for implementation of three central sector schemes of National health program for Rabies, Leptospirosis and Intersectoral coordination program for prevention & control of zoonotic diseases.
The National Rabies Control Program has strengthened the 5 institutes as a Rabies Diagnosis Laboratories. Till date, 9 regional laboratories are strengthened, and labs are mapped in the country currently having a diagnostic facility for rabies. Monitoring and Surveillance in the states has been improved.
- Centre for Environmental, Occupational Health and Climate Change & Health
This centre addresses the health-related issues pertaining to climate and environmental factors. After introduction of “Mission on Health” in year 2015 under the Prime Minister’s Council on Climate Change (PMCC), India’s National Action Plan for Climate Change and Human Health (NAPCCHH) was prepared and to implement it National Programme on Climate Change and Human Health (NPCCHH) under National Health Mission (NHM) was approved by MoHFW in February 2019; this centre looks into NPCCHH operations in the country. Key objectives are increasing awareness, building capacity of health professionals, strengthening health systems and infrastructure for response – health adaptation plans, vulnerability assessments, surveillance, EWARS, etc, building collaborations and research & innovations in context of climate change and human health.
- Centre for Non-Communicable Diseases
In response to the increasing burden of non-communicable diseases, Centre for Non-Communicable Diseases (NCDs) was set up in February 2015, in NCDC with the objectives of providing technical support to NPCDCS, capacity building, IEC & advocacy with policy makers & NPCDCS programme managers, monitoring & evaluation and research. Officers of the division are members of committee for the project “PRATAP” (Programme for Risky behaviour and Attitude in Trauma Prevention)
15. National Viral Hepatitis Control Program (NVHCP)
National Viral Hepatitis Control Program under the National Health Mission in alignment with SDG 3.3 aims to target the management of 5 crore people possibly harbouring the infection. Under the program, free diagnostics and drugs are being made available to all in need, not only for treatment of hepatitis C, but also for life-long management of hepatitis B. The key strategies adopted under the program include preventive, promotive and curative interventions with the focus on awareness generation, increasing access, promoting diagnosis and providing treatment for viral hepatitis. During 2018–21(till Sept 2021), it benefitted nearly 1.5 crore individuals and treated more than 94,000 patients of viral hepatitis
Currently, services for the diagnosis and treatment of viral hepatitis are available in all the states and UTs. The programme has collaborated with the National Program for Surveillance of Viral Hepatitis (NPVSH), National AIDS Control Program and Immunization Division. NVHCP has a national helpline (1800-11-6666) along with the TB helpline to provide access to information about viral hepatitis and services under the programme. The Programme is also in process of collaborating with other existing programs such as RMNCHA+N etc. for effective implementation of the program. The programme has the paperless data recording & reporting on NVHCP Management Information System for robust Monitoring & Evaluation.
During COVID-19, programme initiated multi-month drug dispensations and facilitated issuance of movement passes to the beneficiaries for better compliance and access to health care services under NVHCP.
Achievements (July, 2018 – September, 2021):
- 866 treatment sites established for management of viral hepatitis across 701 districts.
- Serological tests done for diagnosis of viral hepatitis C: 46,22,492
- No. of patients initiated on treatment of Hepatitis C : 84,349
- Patients who have completed the treatment of HCV (end of treatment): 35,040
- Serological tests done for diagnosis of viral hepatitis B: 1,12,27,134
- No. of patients initiated on treatment of Hepatitis B: 10,167
16. Central Government Health Services (CGHS)
CGHS is providing healthcare facilities to 13.76 Lakh primary Card holders ( and 39.79 Lakh- total beneficiaries) through a network of 333 Allopathic Wellness Centres and 95 Ayush Centres/ units located in 74 Cities across India.
17.1 Opening of New Allopathic Wellness Centers/ AYUSH Units
Opening of New Allopathic Wellness Centers during the year at
- Upgradation of Extension Counter at Wadi, Nagpur
- Upgradation of Extension Counter at Ishapore, Kolkata
Approval of the Department of Expenditure has been received for opening 10 more new Allopathy Wellness Centres.
Opening of new AYUSH Units
Ayurvedic Units at Raipur ,Jabalpur, Bhopal and Gandhi Nagar
Homeopathy at Bhubaneswar and Gurgaon.
17.2 Other Achievements:
- CGHS Medical Officers and Staff have been part of the fight against COVID-19 Infection — performing duties at Air-ports and Quarantine Centres.
- Special Provisions to CGHS beneficiaries in view of the COVID-19 Infection:
- Option to purchase OPD Medicines for Chronic illnesses till 31st October 2021 and claim reimbursement
- Directions to open separate ‘Fever Clinic’ at Wellness Centres for screening beneficiaries for Fever and other suggestive symptoms and referral to Nodal Centres
- Directions to CGHS Wellness Centres to provide assistance to COVID 19 +ve CGHS beneficiaries under Home Quarantine and permission to such CGHS beneficiaries to purchase one Pulse Oxymeter (@ Rs1200/-) per family
- Tele- consultation facility with Govt. Specialists through e-Sanjeevani
- Online payment of subscription through `Bharatkosh’
- Fortnightly webinar on health topics for providing health education to CGHS beneficiaries
- CGHS acted as COVID Vaccination Centres(CVC) at identified CGHS Wellness Centres as per directions of Local Health Authorities
- CGHS doctors were deputed to hospitals temporarily on the request of Local health Authorities.
17.3 Settlement of Hospital Bills:
Special Attention to settlement of Hospital Bills to ensure availability of Liquidity with private hospitals empanelled under CGHS so that they extended facilities to CGHS beneficiaries , particularly pensioners.
Hospitals bills of about Rs.1100 Cr are cleared during the current financial year till date.
Settlement of Hospital bills under CGHS has been migrated to NHA IT Platform for settlement of bills in paperless mode.
17. Drug Regulation
- National Centre for Cell Science (NCCS), Pune and National Institute of Animal Biotechnology (NIAB), Hyderabad under the Department of Biotechnology have been notified vide S.O. 2609(E), dated 28.06.2021 and S.O. 3364(E), dated 17.08.2021 respectively as Central Drugs Laboratory (CDL) for a period of one year for testing and lot release of COVID-19 vaccine in order to ease the workload of CDL, Kasauli. National Institute of Biologicals (NIB), Noida has again been notified vide S.O. 5139(E), dated 10.12.2021 as CDL for a period upto 30.11.2022.
- Standalone Bio-analytical Laboratory has been brought under the regulation of New Drugs and Clinical Trials Rules, 2019 vide notification No. G.S.R. 605(E), dated 31.08.2021.
- Rule 90 of the Drugs Rules, 1945 has been amended vide notification no. G.S.R. 766(E), dated 27.10.2021 to set timelines of 7 working days to issue test licenses in Form 29 under the Union Government initiative of “Reducing Compliance Burden”.
- Rule 24 and rule 24 A of the Drugs Rules, 1945 have been amended vide notification no. G.S.R. 839(E), dated 29.11.2021 in order to reduce redundancy in the regulation and the same is a step toward reducing compliance burden.
- Tapentadol, centrally acting opioid analgesic drug, has been brought in the list of Schedule H1 of the Drugs Rules, 1945 vide notification no. G.S.R. 258(E), dated 07.04.2021 as per recommendations of Chairperson, National Commission for Protection of Child Rights in order to keep it out of the reach of Children and Adolescents.
18. DENTAL COLLEGES
At present, there are 316 dental colleges in the country, out of which 50 are in Government Sector and 266 in private sector with annual admission capacity of about 27498 Under Graduate seats and 6696 Post Graduate seats. Under EWS scheme promulgated under 103rd constitutional amendment, 2019, the DCI has recommended 498 additional seats at UG level at 30 Govt. Dental Colleges and 43 PG Seats at 15 Govt. Dental Colleges for the academic session 2021-22.
REFORMS IN DENTAL EDUCATION:
THE DRAFT NATIONAL DENTAL COMMISSION BILL
The Government has initiated a series of reforms in the sector of medical education over the last six years. As part of these reforms and in order to revamp the Dental Education System in the country to bring it at par with global standards, a draft National Dental Commission Bill (the Bill), to replace the existing Dentists Act, 1948, has been prepared and was placed in public domain on 28.01.2020 for inviting comments of State Governments, general public and other stakeholders. The 2372 suggestions/comments received from various stakeholders have been examined by a Committee of Dental Experts under the Chairpersonship of Chief of the Centre for Dental Education & Research, AIIMS, New Delhi. The suggestions of the committee have been duly considered and incorporated appropriately in the Bill.
The Bill provides for the constitution of a National Dental Commission (the Commission), four Autonomous Boards, a Dental Advisory Council and State Dental Councils. The Commission shall be a corporate body. The Bill further provides for constitution of four Autonomous Boards namely Undergraduate Dental Education Board (UGDEB), Postgraduate Dental Education Board (PGDEB), Dental Assessment and Rating Board (DARB) and Ethics and Dental Registration Board (EDRB) – to regulate the education, examination, training and services of dental professionals and dental auxiliaries like dental hygienists, dental technicians and dental operating room assistants. A provision for National Dental Advisory Council has also been kept in the Bill to have representation of States/UTs at National level. The Bill also provides for creation and maintenance of digital and live National and State Registers of all licensed dental professionals and dental auxiliaries.
As per the Bill, the Commission shall lay down policies for maintaining high standards in dental education, and shall also exercise appellate jurisdiction with respect to the decisions of the Autonomous Boards. The Commission shall frame guidelines and conduct a uniform entrance test at undergraduate level i.e. National Eligibility cum Entrance Test, a uniform exit test i.e. National Exit Test (Dental), common counselling for admission to undergraduate and postgraduate dental courses etc.
The Bill empowers the Central Government to give directions to the Commission and the Autonomous Boards on questions of policy and also to the State Governments for carrying out all or any of the provisions of this Act.
The draft Bill is still under consideration.
19. National Programme for Health Care of the Elderly (NPHCE)
The Government of India had launched the “National Programme for Health Care of the Elderly” (NPHCE) during 2010-11, to address health related problems of elderly people and to provide separate, specialized and comprehensive health care to the senior citizens at various levels of state health care delivery system including outreach services. Preventive and promotive care, management of illness, health manpower development for geriatric services, medical rehabilitation & therapeutic intervention and IEC are some of the strategies envisaged in the NPHCE. During 2020-21, a total number of 725 districts of 35 States/UTs and 19 Regional Geriatric Centres (RGCs) and 02 National Centres for Aging (NCAs) are sanctioned under the programme. Of the 725 districts sanctioned,595 district hospitals & 18 RGCs have operationalised for NPHCE services. The programme implementation at States/UTs level is through funds under NCD Flexible pool of National Health Mission (NHM) and at Government Medical Colleges/Institutes through funds under Tertiary care programme activities of Ministry of Health & Family Welfare.
Progress in Operationalization of the Programme activities 2021:-
(As per Progress Report- April to Sep., 2021)
|
S. No |
Institutions |
Sanctioned |
Operational |
|||
|
OPD |
Indoor wards |
Physiotherapy services |
Laboratory services |
|||
|
1 |
RGCs |
19 |
18 |
16 |
14 |
13 |
|
2 |
District hospitals |
725 |
595 |
510 |
459 |
540 |
|
3 |
CHCs |
4875 |
3111 |
– |
1131 |
2439 |
|
4 |
PHCs |
18444 |
10231 |
– |
– |
– |
|
5 |
SCs providing home based care & supportive appliances |
90932 |
14320 |
|||
Daily Geriatric OPD services are being provided in 595 DH, 3111 CHCs and 10231 PHCs along with special OPDs in 18 RGCs. Inpatient services are being provided in 510 DH, along with 16 RGCs. Physiotherapy services are being provided in 459 DH, 1131 CHCs along with 14 RGCs.Laboratory services are being provided in 540 DHs, 2439 CHCs, along with 13 RGCs.
Training Modules: Three sets of Training modules for Medical Officers, Nurses and Community based workers to deliver Comprehensive Geriatric Assessment & Care have been developed and shared with all States/UTs. The State level Training of Trainers of Medical Officers & Nurses for Comprehensive Geriatric Assessment & Care is being conducted.
IEC:-Audio/Video spots on different topics of elder care, print material-folder, posters etc. have been developed. The regional language version of IEC material is being developed. https://nphce.nhp.gov.in/video-spot/
Release of Longitudinal Ageing Study in India (LASI) WAVE-I Report:-The LASI is a nationally representative survey of older persons in India is being undertaken through IIPS, Mumbai. LASI wave-1 survey (2017-18) covers all 30 states and 6 Union Territories of India with a panel sample size of 72,250 older adults aged 45 years including 31464 people above 60 years of age and above and their spouses regardless of age. LASI collects data on four major subject domains:
- Health: Disease Burden & Risk Factors (Reported and Measured)
- Health Care and Health Care Financing
- Social: Family, Social Network and Social Welfare Programmes for the Elderly
- Economic: Income, Wealth, Expenditure, Employment, Retirement and Pension
The first wave of LASI has been completed and the final report of LASI wave-I released by Hon’ble Union Health Minister on 6th January 2021.Longitudinal Ageing Study in India- LASI Wave-1 Report along with India & States/UTs Fact Sheets is available at-
Workshop to address Comprehensive Geriatric Care Assessment & Delivery through Various National Health Programmes (Virtual):-
During the release of LASI WAVE -1 Report an Exploratory Workshop to understand the various National Health Programmes for delivering Comprehensive Geriatric Care at various level of service delivery followed, was conducted with the expert groups and the National Programmes; National Program for Control of Blindness and Visual Impairment (NPCBVI), National Program for Prevention and Control of Deafness (NPPCD), National Program for prevention and control of Cancer, Diabetes, Cardiovascular Diseases and Stroke (NPCDCS), National Oral Health Programme (NOHP), National Mental Health Program (NMHP) and National Programme for Palliative Care (NPPC).These National Health Programmes are providing few facilities for geriatric care under their programmes. Subsequently six different workshops (Programme wise) also planned to conduct for development of collaborative implementation framework for elderly care services. A collaborative workshop between NPHCE & NPCBVI was conducted on 3rd June 2021. 




Performance Review of the Tertiary Care Services:- The Review meeting of Tertiary care activities of the National Program for Healthcare of Elderly (NPHCE) was held under the chairpersonship of Dr. Anil Kumar, DDG (IH) to review the physical & financial progress of the Regional Geriatric Centers (RGCs) and two National Centers for Ageing (NCAs).
The review meetings were attended by Dr. Rupali Roy, ADG (IH) Dte. GHS, Dr. Avinash Sunthlia, MO (IH) Dte. GHS and Ms. Anita Ahuja, Consultant (Training & IEC)NPHCE. These meetings were conducted in 5 groups and Nodal officers of concern RGCs participated as per schedule from 16th July to 2nd September 2021. The purpose of the review meeting was;
- Review functioning of Regional Geriatric Centers (RGCs)
- Assess physical progress of the RGCs in terms of services delivery
- To discuss the overall issues and issues related to initiating MD geriatrics
- Plans for expansion of the activities of the NPHCE
1stOctober-International Day for Older Persons:-Every year 1st October is celebrated as the International Day of Older Persons to recognize the important contributions that older people make to our world, while raising awareness towards issues of aging. The theme of the International Day of Older Persons 2021 is: “Digital Equity for All Ages’’. One day event
Multi-Stakeholder Dialogue on Elderly care & Digital Equity for All Ages Was conducted byCentral Programme Division of National Programme for Health Care of Elderly (NPHCE)in collaboration with WHO India on 1st October 2021 at Hotel Park, New Delhi with 100 participants from socio-economic strata of geriatric population.



Activities Conducted by States/UT to commemorate International Day for Older Persons:-
- Health Camps for elderly with COVID protocols and Distribution of Aids procured through the social welfare department.
- Awareness Programme on Healthy Ageing, NCD Screening, Demonstration of Physical exercise/Yoga, Distribution of Elderly Diaries, Counselling sessions etc.
- Felicitation of the elderly person in various States including NGOs & Institutions.
- Awareness generation through Hoardings at prominent places, wall paintings, posters, pamphlets, Radio programmes, announcements, News Paper Advertisement, community programs through IEC van.
- Sensitization /workshop for all working staff for implementation of NPHCE programme at District and CHC level.
- Month long campaign to broadcast awareness messages regarding available services of NPHCE, Awareness Activities at old age homes and PHC level.
*******
MV

