
: Mumbai, December 29, 2023
Doctors from Tata Memorial Hospital, Mumbai, and the Advanced Centre for Training Research and Education in Cancer (ACTREC), Navi Mumbai, collaborated with IDRS Labs, Bangalore, to develop the first and only oral suspension of 6-mercaptopurine (6-MP) in India. 6-MP is a chemotherapy drug used in the treatment of Acute Lymphoblastic Leukemia (ALL), the most common type of blood cancer afflicting children. The child friendly formulation is available in the form of a Powder for Oral Suspension, and is marketed under the tradename PREVALL.
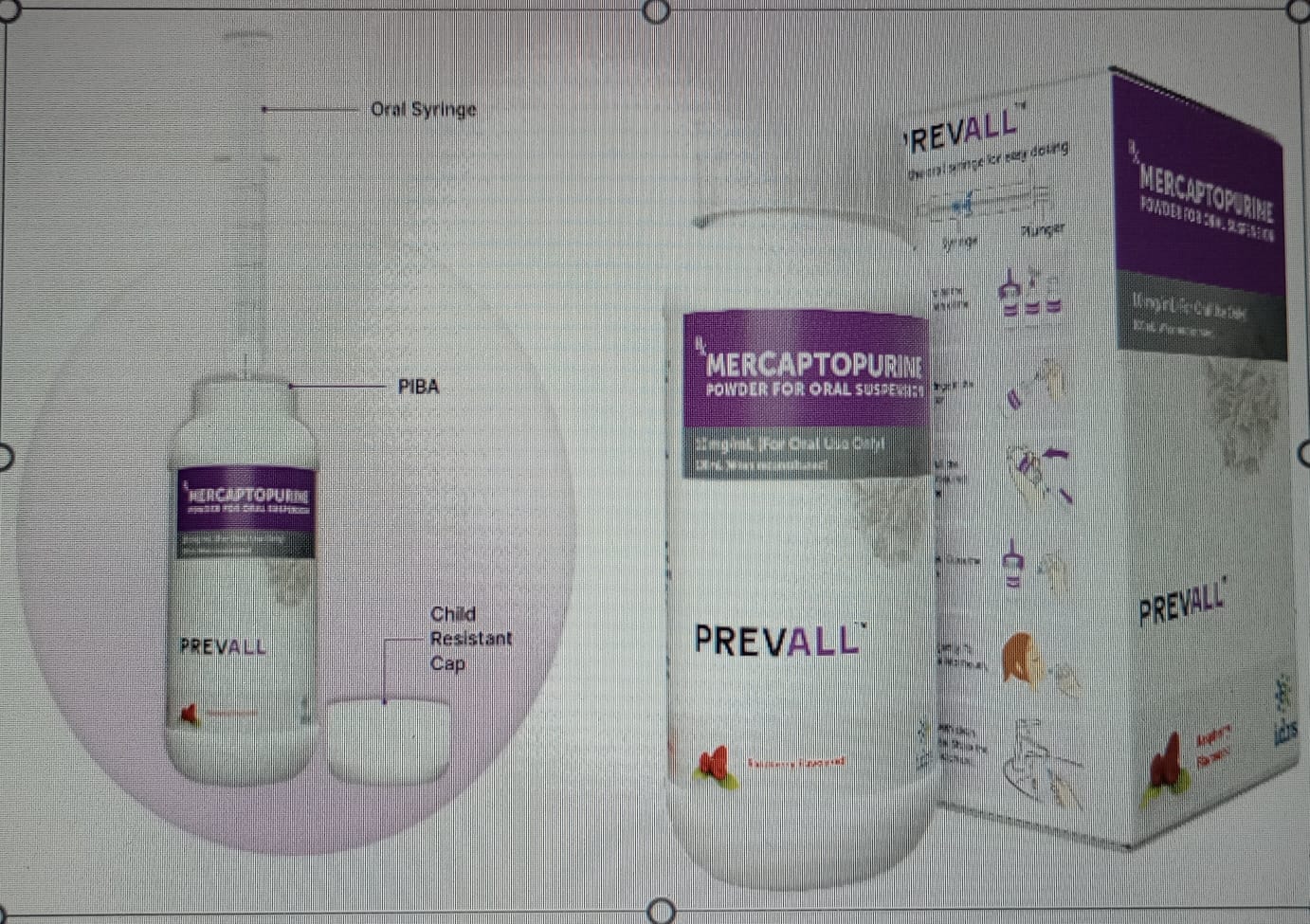
PREVALL can be effortlessly reconstituted into a 100 ml oral suspension at a concentration of 10mg/ml. PREVALL is accompanied by a syringe and a Press In Bottle Adapter (PIBA) that allow precise dosing tailored to a patient’s body weight or body surface area. These features not only aid in accurate administration but also mitigate the risk of spillage and caregivers’ exposure to cytotoxic compounds.
The introduction of PREVALL marks a significant milestone, addressing several challenges posed by the current tablet formulation in terms of dosage accuracy, flexibility, and tolerability. Hitherto, suboptimal practices such as crushing the tablet or alternate day dosing were being adopted to meet the dosing requirements in children.
Regulatory approval: PREVALL has received approval from Central Drugs Standard Control Organization (CDSCO), the national drug regulatory body under the Ministry of Health and Family Welfare, Government of India. This regulatory clearance emphasizes the safety and compliance of PREVALL, providing assurance to both healthcare professionals and patients about its efficacy and quality. Tata Memorial Centre and IDRS Labs jointly published the results of the clinical study leading to regulatory approval in the scientific journal Pediatric Blood and Cancer recently.
Market Availability: The dry powder pharmaceutical suspension of 6-mercaptopurine is a patented technology of IDRS Labs Private Limited. IDRS Labs officially launched PREVALL at the PHOCON conference in Chennai on 25th November, 2023. The formulation was introduced in Tata Memorial Hospital, Mumbai, in early December, 2023, and shall be available in all major hospital pharmacies across the country very soon. Approximately 10,000 children in the age group of 1-10 years diagnosed with ALL are expected to benefit from PREVALL each year.
Dr. Girish Chinnaswamy, Professor & Head, Department of Paediatric Oncology, Tata Memorial Hospital, commented that children with curable cancers such as ALL deserve the best possible care, and formulations such as PREVALL would help in ensuring dose optimization, improving adherence, and maximizing the efficacy of drugs.
Dr. Vikram, Professor of Clinical Pharmacology, ACTREC, observed that PREVALL is a manifestation of the inherent strength and depth of Indian biopharma, conceiving a formulation best suited for the Indian conditions. “The powder for oral suspension is designed to ensure stability of the drug in hot/humid conditions and is quite distinct from the liquid formulation available elsewhere in the world” he noted.
Dr. Banavali, Director-Academics, Tata Memorial Centre, Mumbai, and a senior paediatric hemato-oncologist commented that PREVALL has accomplished an unmet medical need in paediatric hemato-oncology. He said liquid formulations of 6-Mercaptopurine is available in Europe and USA for a long time, and it is only fair that children in India and other developing countries have access to products that are standard of care in the developed world.
Dr. Sudeep Gupta, Director, Tata Memorial Centre, Mumbai, commended the fine collaborative effort and emphasized the need for more such collaborations between the academia and industry to improve access to novel drugs and formulations. He said Tata Memorial Centre is committed to fostering innovation, citing the example of CAR-T cell therapy which was also pioneered by Tata Memorial Centre recently in a similar collaborative effort.
Dr. Ajit Kumar Mohanty, Secretary Department of Atomic Energy and Chairman Atomic Energy Commission remarked that the development is an outcome of the convergence of academia and industry interests to bridge the gap between unmet need and innovation in healthcare. He also mentioned that this is in line with department’s initiative of bringing innovation from lab to land for the benefit of society.
SC/PM Source: DAE
Follow us on social media:![]() @PIBMumbai
@PIBMumbai  /PIBMumbai
/PIBMumbai ![]() /pibmumbai
/pibmumbai  pibmumbai[at]gmail[dot]com
pibmumbai[at]gmail[dot]com  /PIBMumbai
/PIBMumbai  /pibmumbai
/pibmumbai

